





AQA AS level Unit 1 section 2 Amount of substance complete lesson package, homework and assessments
Using the specification and books
No exam questions are included due to copy right (unless written out by myself)
Including:
Homework booklets
Assessment sheets
Interactive powerpoints (rarely seen in A-level)
STUDENT WORKBOOK FOR REQUIRED PRACTICAL FOUND HERE IF REQUIRED
-
Calculation of reacting volumes of gas (EXTRA LESSON - removed from spec)
-
Empirical and Molecular formulea
-
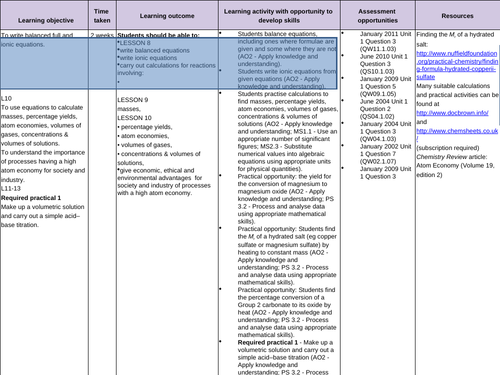
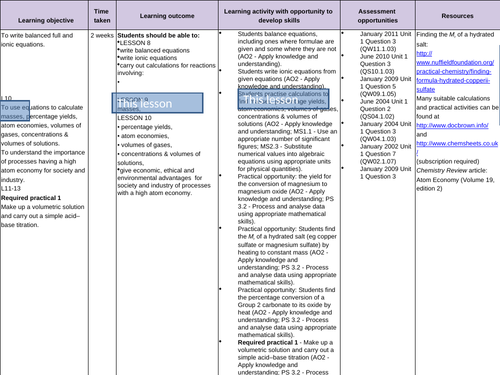
Balancing equations and Ionic equations
-
Reacting masses
-
Atom economy and percentage yield
-
EXTRA LESSON - Limiting reagents (student support IF REQUIRED)
-
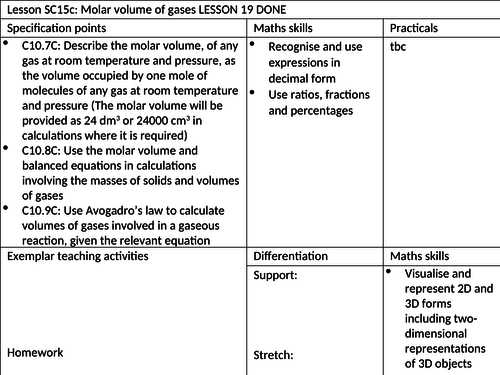
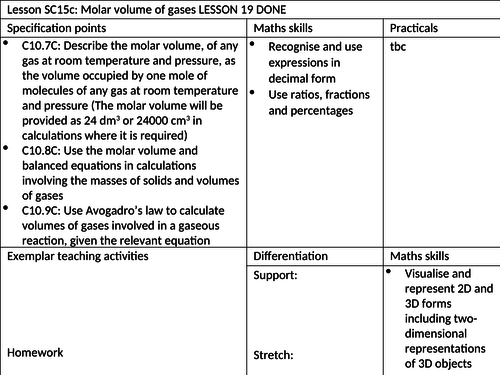
To know how to define molar volume of gases at room temperature and pressure
To be able to use the molar volume in calculations involving the masses of solids and volumes of gases
To understand how to use Avogadro’s law to calculate volumes of gases involved in gaseous reactions. -
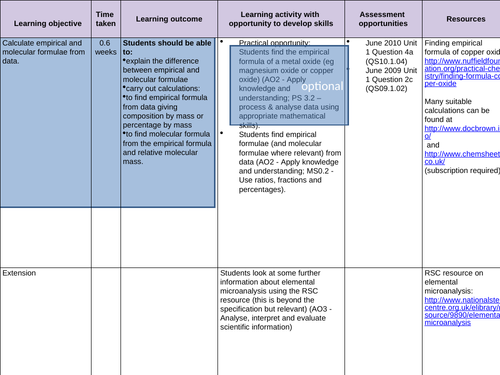
To know the terms molecular and empirical formula
To be able to deduce the molecular formula of a compound from its empirical formula and its relative molecular mass
To find empirical formula from data giving composition by mass or percentage by mass -
To recall how to balance equations
To be able to separate aqueous compounds into ions in order to write ionic equations
To be able to write balanced ionic equations -
To complete a 10 question check up
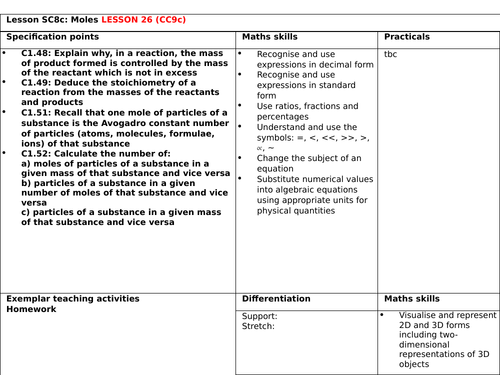
To be able to calculate the amount of product from a given reactant
To know how to consolidate learning with questions after completing a practical
10.To understand the difference between the actual yield and the theoretical yield
To be able to calculate the percentage yield of a reaction from the actual yield and the theoretical yield using moles
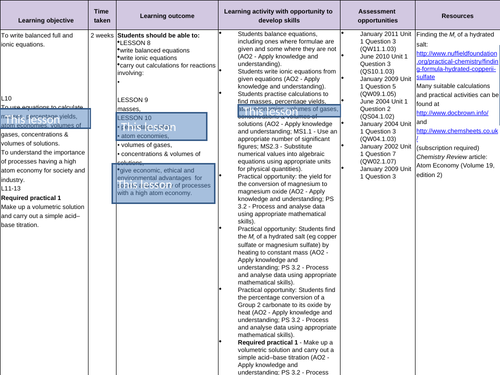
To calculate atom economy
- Industrial processes
- To understand the term ‘limiting reagent’
To be able to identify the limiting reactant in a reaction
To be able to calculate the mass of the reactant from the limiting reactant using moles
Get this resource as part of a bundle and save up to 41%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
AQA AS level Unit 1/Section 1: Physical chemistry COMPLETE (lessons and worksheets) - atomic structure, amount of substance, bonding, energetics, kinetics, equilibria, REDOX
AQA AS level Unit 1 Section 1 Atomic structure (atom, electrons, mass spec, ionisation energies) Including: Homework booklets Assessment sheets Interactive powerpoints (rarely seen in A-level) SECTION 1: Atomic structure 1. FUNDAMENTAL PARTICLES - The atom 2. Atomic models (developing ideas from GCSE) 3. Relative mass, relative atomic mass and atomic number 4 Mass spectrometer 5. Mass spectrum analysis - using mass spectra 6. Electron structure - shells and sub-level (s, p, d, f) 7. Ionisation energies - trends and equations SECTION 2: Amount of Substance 14 lessons in total 1.Masses and Mole Part 1 2.Masses and Mole Part 2 3. Moles in solution 4. Ideal Gas equation part 1 5. Ideal Gas equation part 2 - DEMO 6. Calculation of reacting volumes of gas (EXTRA LESSON - removed from spec) 7. Empirical and Molecular formulea 8. Balancing equations and Ionic equations 9. Reacting masses 10. Atom economy and percentage yield 11. EXTRA LESSON - Limiting reagents (student support IF REQUIRED) 12. Standard solutions 13. Titrations 1 14. REQUIRED PRACTICAL 1 Making a standard solution SECTION 3: Bonding 1. Ionic bonding 2. Metallic bonding 3. Covalent bonding 4. Dative covalent (co-ordinate) bonding 5 + 6 Shapes of molecules 7 Electronegativity and bond polarity 8 + 9 Forces acting between molecules (van de Waals, dipole-dipole and Hydrogen bonding) 10 States of matter and a summary of 4 types of crystal structure - molecular, macromolecular, ionic and metallic SECTION 4: Energetics 1. Endothermic/exothermic 2. Measuring q (Measuring Enthalpy Change) 3. PRACTICAL CHOICES 4. Enthalpy of Formation 5. Enthalpy of Combustion 6. Required Practical 7. Bond Enthalpy SECTION 5: KINETICS 1. Collision theory and rates (GCSE RECAP) 2. Maxwell-Boltzmann distribution 1 3. Maxwell-Boltzmann distributions 2 4. REQUIRED PRACTICAL 3 5. Catalysts SECTION 6: Equilibria 1. Dynamic equilibrium + Le Chatelier’s principle 2. Equilibrium and Industry 3. Equilibrium Constant, Kc 4. Kc - calculating moles and composition 5. To predict the effect, if any, of the changes in conditions on the value of Kc SECTION 7: RedOx 1. ‘oxidation’ and ‘reduction’ and oxidation states 2. and 3. 1/2 equations (oxidising agents and reducing agents) 4. Optional practicals FOR MORE INFORMATION SEE EACH INDIVIDUAL UPLOAD Save 37% buying in bulk
AQA AS level Unit 1 Section 2: Amount of substance complete lesson package, homework and assessments
Using the specification and books No exam questions are included due to copy right (unless written out by myself) Including: Homework booklets Assessment sheets Interactive powerpoints (rarely seen in A-level) STUDENT WORKBOOK FOR REQUIRED PRACTICAL FOUND HERE IF REQUIRED https://www.tes.com/teaching-resource/aqa-a-level-chemistry-required-practical-tracking-booklet-competencies-12051709 14 lessons in total 1.Masses and Mole Part 1 2.Masses and Mole Part 2 3. Moles in solution 4. Ideal Gas equation part 1 5. Ideal Gas equation part 2 - DEMO 6. Calculation of reacting volumes of gas (EXTRA LESSON - removed from spec) 7. Empirical and Molecular formulea 8. Balancing equations and Ionic equations 9. Reacting masses 10. Atom economy and percentage yield 11. EXTRA LESSON - Limiting reagents (student support IF REQUIRED) 12. Standard solutions 13. Titrations 1 14. REQUIRED PRACTICAL 1 Making a standard solution 1. To state the definition for relative atomic mass in terms of carbon-12 and calculate Ar To state the definition for relative molecular mass in terms of carbon 12 and calculate Mr To understand the concept of the mole and Avogadro’s constant 2. To understand the term molar mass To use Avogadro constant to calculate the number of atoms or molecules To use Avogadro constant to calculate the number of molecules or atoms from mass 3. To understand the term concentration To be able to calculate concentrations in a given volume of solution To be able to calculate the concentration in mol dm-3 from the mass 4. To understand the gas laws To recognise and make use of appropriate units in ideal gas calculations To carry out calculations using the ideal gas equation 5. To complete a practical to find the relative molecular mass of the lighter fuel To find the relative molecular mass of the lighter fuel using the ideal gas equation To complete exam questions 6. To know how to define molar volume of gases at room temperature and pressure To be able to use the molar volume in calculations involving the masses of solids and volumes of gases To understand how to use Avogadro’s law to calculate volumes of gases involved in gaseous reactions. 7. To know the terms molecular and empirical formula To be able to deduce the molecular formula of a compound from its empirical formula and its relative molecular mass To find empirical formula from data giving composition by mass or percentage by mass 8. To recall how to balance equations To be able to separate aqueous compounds into ions in order to write ionic equations To be able to write balanced ionic equations 9. To complete a 10 question check up To be able to calculate the amount of product from a given reactant To know how to consolidate learning with questions after completing a practical 10.To understand the difference between the actual yield and the theoretical yield To be able to calculate the percentage yield of a reaction from the actual yield and the theoretical yield using moles To calculate atom economy + Industrial processes 11. To understand the term ‘limiting reagent’ To be able to identify the limiting reactant in a reaction To be able to calculate the mass of the reactant from the limiting reactant using moles 12. To recall ways in which neutralisation can occur To know the term standard solution To understand how to make a standard solution 13. To understand how to carry out an acid and alkali titration To be able to carry out a titration To be able to carry out calculations using the results of titrations to calculate an unknown concentration of solution or unknown volume of solution 14. Required practical 1 Make up a volumetric solution and carry out a simple acid–base titration ENJOY! AQA AS level Unit 2 Amount of substance complete lessons, homework + assessments REQUIRED PRACTICAL
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.