





This is an engaging revision lesson which uses a range of exam questions, understanding checks, quiz tasks and quiz competitions to enable students to assess their understanding of the content within topic 9 (The Periodic Table) of the Cambridge IGCSE Chemistry (0620) specification. The lesson covers the content in both the core and supplement sections of the specification and therefore can be used with students who will be taking the extended papers as well as the core papers.
The specification points that are covered in this revision lesson include:
CORE
- Describe the Periodic Table as a method of classifying elements and its use to predict properties of elements
- Describe the change from metallic to nonmetallic character across a period
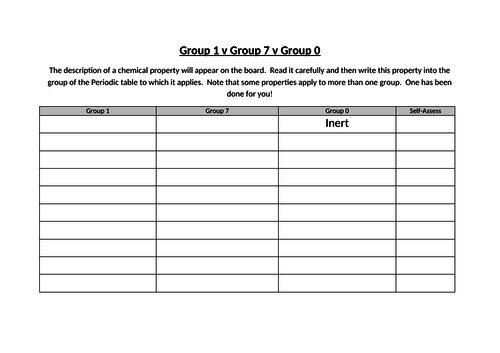
- Describe lithium, sodium and potassium in Group I as a collection of relatively soft metals showing a trend in melting point, density and reaction with water
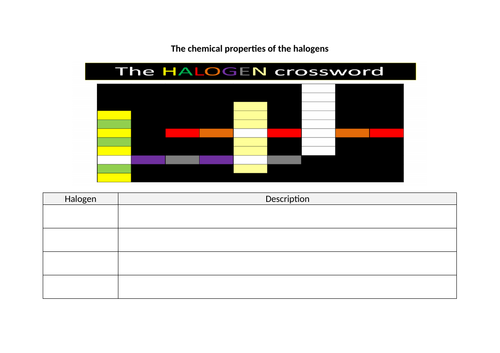
- Describe the halogens, chlorine, bromine and iodine in Group VII, as a collection of diatomic non-metals showing a trend in colour and density and state their reaction with other halide ions
- Predict the properties of other elements in Group VII, given data where appropriate
- Describe the transition elements as a collection of metals having high densities, high melting points and forming coloured compounds, and which, as elements and compounds, often act as catalysts
- Describe the noble gases, in Group VIII or 0, as being unreactive, monoatomic gases and explain this in terms of electronic structure
- State the uses of the noble gases in providing an inert atmosphere, i.e. argon in lamps, helium for filling balloons
SUPPLEMENT
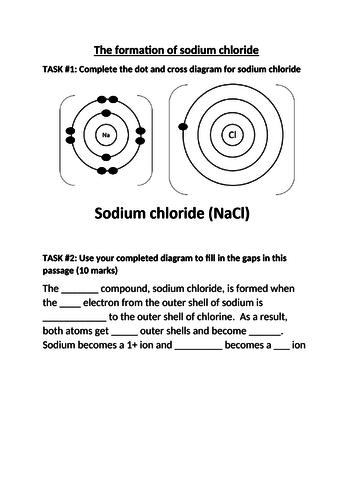
- Describe and explain the relationship between Group number, number of outer shell electrons and metallic/non-metallic character
- Identify trends in Groups, given information about the elements concerned
- Know that transition elements have variable oxidation states
The students will thoroughly enjoy the range of activities, which include quiz competitions such as “Make sure you check every passage PERIODICALLY” where they have to scan summary passages about the table and decide if it is 100% correct whilst crucially being able to recognise the areas of this topic which need their further attention. This lesson can be used as revision resource at the end of the topic or in the lead up to mocks or the actual GCSE exams
Get this resource as part of a bundle and save up to 50%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.