

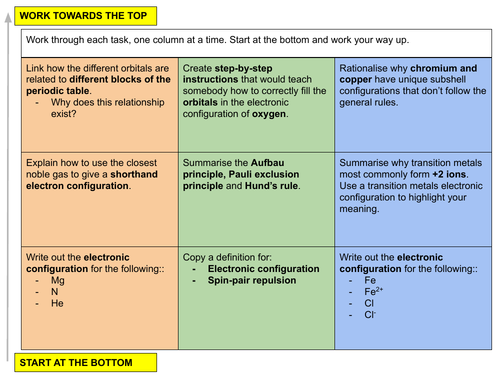
In this lesson we focus on the rules stating how electrons fill orbitals and how to write electron subshell configuration. This is lesson six in our physical chemistry series for Unit 2: Atomic Structure (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
LESSON OBJECTIVE: Understand how electrons fill orbitals and to determine subshell electronic configuration for given atoms and ions.
LEARNING OUTCOMES (from the Cambridge AS Chemistry Curriculum 2019-2021):
2.3 Electrons: energy levels, atomic orbitals, ionisation energy, electron affinity
c) state the electronic configuration of atoms and ions given the proton (atomic) number and charge, using the convention 1s22s22p6, etc.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.