Equilibrium- Higher tier
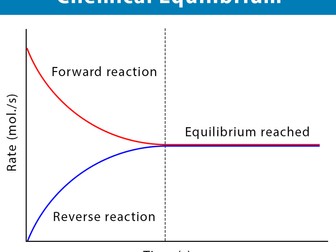
Dynamic equilibrium explained
Forward and reverse reactions
Closed systems
Rate of reaction equality at equilibrium
Le Chatelier’s Principle
Effect of concentration changes
Effect of pressure changes (gaseous systems)
Effect of temperature changes
Predicting direction of shift
Applying reasoning to unfamiliar reactions