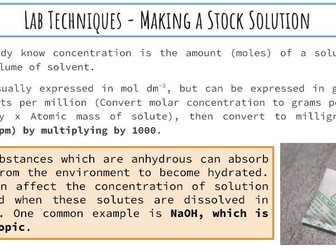
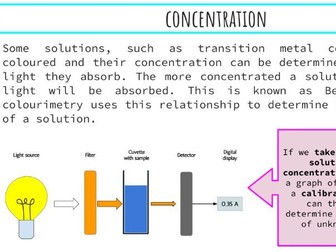
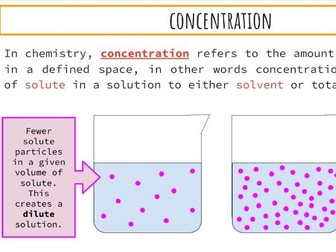
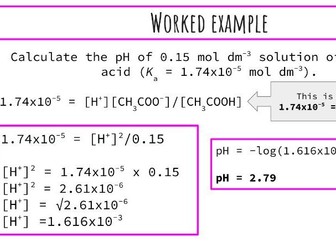
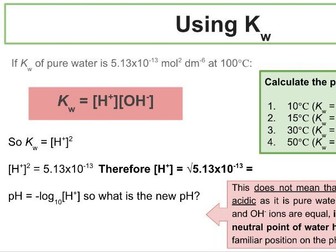
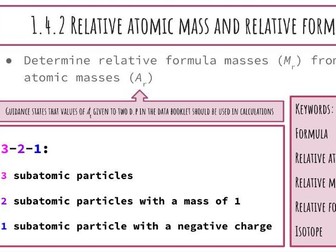
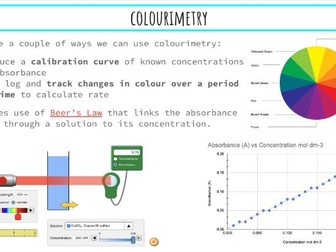
Activation Energy of UV Beads
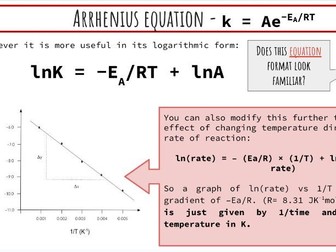
This presentation and student practical sheet introduces the Arrhenius Equation and how it can be modified to determine activation energy (Ea) for a simple experiment using UV beads.
The advantage of this task is it is far more straightforward for students to complete compared to more complex class practicals such as the reaction between bromate and bromide ions or the iodine clock reaction. There is also much less prep and tidying!
This activity includes a spreadsheet of sample data and calculation which can be shared with students.