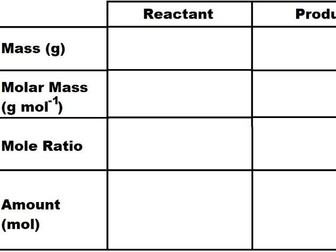
Stoichiometry 2: Percentage Yield and Atom Economy
A resource that can be used as a guide during a lesson or as a revision pack and is the second installment in a series on stoichiometry. This resource focuses on the concepts of percentage yield and atom economy. There are clear definitions and a step-by-step guide on how to calculate the percentage yield and atom economy of a chemical reaction.
This resource is aimed at AS level or equivalent chemistry students to continue on from the previous resource on the topic of stoichiometry.