Can energy be used up? Do food and fuels contain energy?
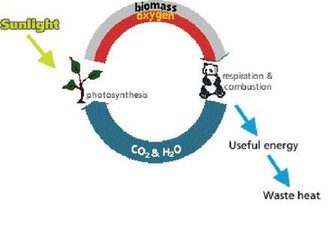
The word energy is a part of our everyday language: it has come to represent an ability to do something useful and is best represented in science lessons as ‘useful’ or ‘high-grade’ energy. As this ‘energy’ is ‘used’ it degrades into waste heat, so we have to come back for more. This contrasts with the idea that the amount of energy, measure in Joules, remains constant.
We also look at why we think that food and fuels ‘contain’ energy (they don’t) – it stresses that oxygen is necessary for energy transfer but is usually ignored because it is invisible and freely available from the air.
This resource is mainly useful as it stands for INSET or teacher education. Modified it can be used in the classroom. Comments on how you have used it would be welcome.