
The periodic table is for elements, but can we make sense of the millions of compounds in our environment? The answer explained in this PowerPoint presentation is to classify them according to their physical properties, the same properties we use when we recycle – 1: metals, 2: ceramics (glass), 3: plastic and organic waste, paper, 4: volatile waste we send up the chimney or down the drain; 5: ionic substances like ash.
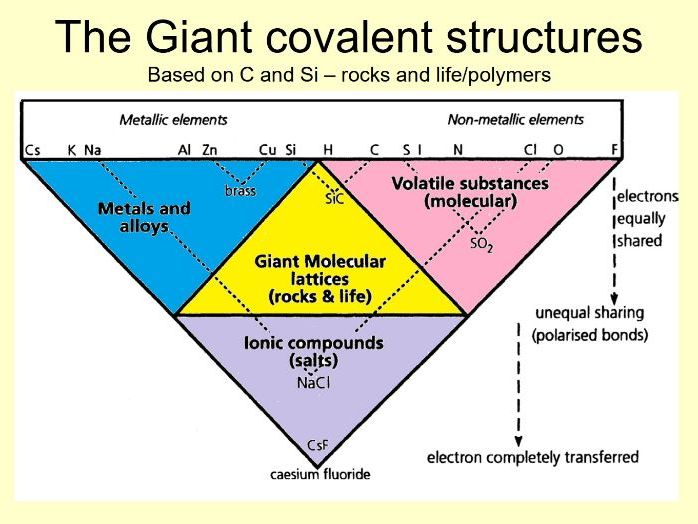
We have just five different sorts of compound. We find the same five groups when we look at the ways elements bond. Forget classifying substances into solid, liquid or gas and go, instead, for these five types: metals, ceramics, life polymers (both biodegradable and plastics), volatile and ionic. We tend to teach bonding as if there are three distinct ‘kinds’: covalent, ionic and metallic, whereas these are just extreme cases. They all start with two atoms sharing electrons.
With two non-metallic elements this results in (polarised) covalent bonding, either building up individual molecules (volatile materials) or building up giant structures (rocks , life and polymers)
with two metallic elements you can never fill the outer electron shells so the atoms close pack with ‘free’ electrons (metals and alloys)
with a metal and non-metal the bond is very polarised and you get ionic bonding (salts etc.)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.