Some lessons for the new AQA C1 spec
Something went wrong, please try again later.
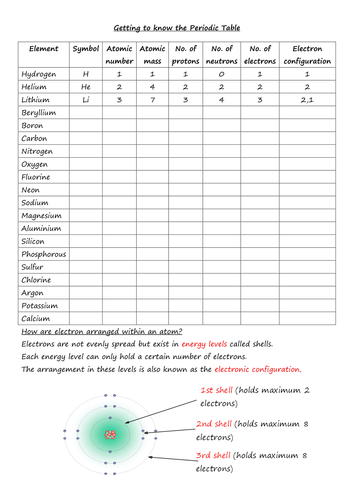
I'm being picky maybe, but the bit in the 'Quiz Wizz' file about 'Sodium donates its one outer electron to chlorine to get a full outer shell' feeds in to a notorious chemical misconception (flagged up in the RSC's resources on the topic). It's true that sodium loses an electron, and it's true that chlorine picks one up from somewhere, but in neither case is the other necessarily (or even usually) involved, and this approach leads to a totally erroneous conception of one sodium ion getting up with one chloride ion after an electron transfer event. This gets in the way of grasping the lattice-like nature of ionic compounds, and how they're built up thanks to electrostatic forces radiating in all directions from every ion. In fact, the same misconception is reflected in the next question, to which the given answer is that the formula of potassium permanganate tells us that 'It contains 1 potassium atom, 1 manganese atom and 4 oxygen atoms chemically bonded together'. Being an ionic compound, what it really tells is just that the three elements involved exist in the ratio 1:1:4. Those oxygen atoms are no more bonded with that one potassium atom than they are with any of the others immediately neighbouring!<br /> <br /> I hope the AQA isn't actively perpetuating this - I don't think I've seen it in the textbook I'm using with the current course. I realise the more correct explanations can be more challenging to explain, but they really do help other concepts make sense and fit together.
Would love to be able to open the first 3 files - any way you could re-upload lesson plan 1 2 and 3 in ppt format? The rest are excellent so would love the full set as I am teaching this section at the moment :)
Could not open all the resources, but those that I did manage to open were excellent. Thank you for sharing.
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.