449Uploads
137k+Views
61k+Downloads
Chemistry

GCSE Chemistry: Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• Filled outer shells result in more stable electronic structures.
• The electronic configuration ionic compounds
• Models of giant ionic structures

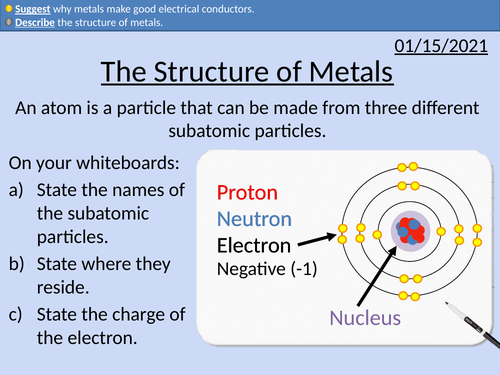
GCSE Chemistry: The Structure of Metals
This PowerPoint presentation with worked examples and student questions covers:
• State a use for metals
• Describe the structure of metals
• Why metals make good electrical conductors.
• Metals on the periodic table

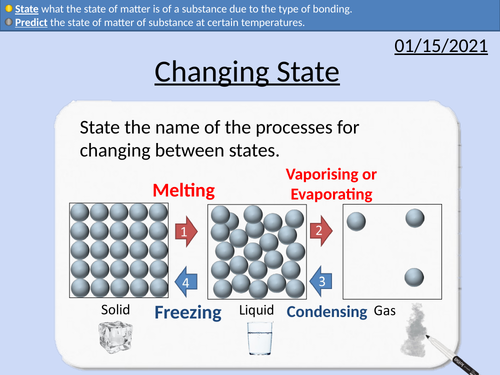
GCSE Chemistry: Changing State
This PowerPoint presentation with worked examples and student questions covers:
• Define melting and boiling point of a pure substance.
• Predict the state of matter of substance at certain temperatures.
• State what the state of matter is of a substance due to the type of bonding.
• Metals, covalent structures, ionic structures and simple molecules.

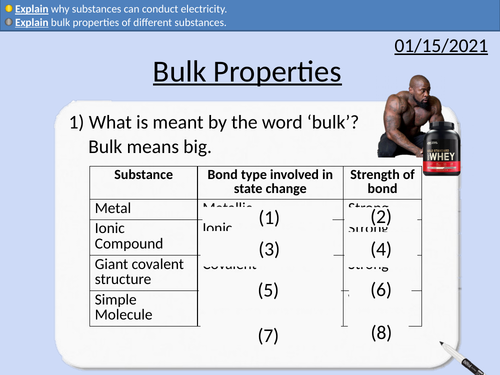
GCSE Chemistry: Bulk Properties
This PowerPoint presentation with worked examples and student questions covers:
• Jobs in Material Science
• Bulk properties of metals - malleable and conductors of electricity
• Bulk properties of ionic and covalent structures - brittle
• Explain why substances conducting electricity depends upon the state of matter

OCR Applied Science: 21.1 Regulatory Bodies
This PowerPoint presentation with worked examples and student activities covers: Topic 1.1 and 1.2 of Module 21: Product Testing Techniques.
Understand the influence of regulatory bodies on development of consumer products.
1.1 The relevant governing bodies that oversee product safety for
manufacturers and consumers of products.
1.2 How governing bodies influence how quality control is applied.

OCR Applied Science: 21.2.2 Testing During Development
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.2 of Module 21: Product Testing Techniques.
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.

GCSE Chemistry: Carbon
This PowerPoint presentation with worked examples and student questions covers:
• State processes of the carbon cycle.
• Define the word allotrope.
• Explain why allotropes have different properties.
• Graphite, graphene, and fullerenes

OCR Applied Science: 21.2.1 Types of Testing
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.1 of Module 21: Product Testing Techniques.
2.1 Types of testing i.e.:
• in-vitro
• in-vivo
• titration
• extraction and separation
Bundle

OCR Applied Science: 21.2 Product Testing of Consumer Products
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
2.1 Types of testing i.e.:
• in-vitro
• in-vivo
• titration
• extraction and separation
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.
2.3 Effectiveness of test i.e.:
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use (e.g. toxicity, possible mutagenic and
teratogenic effects, microbiological safety)

GCSE Chemistry: Nanoparticles
This PowerPoint presentation with worked examples and student questions covers:
• Relative size of nanoparticles
• Convert nanometres using standard form
• Uses and dangers of nanoparticles

GCSE Chemistry: Formulae of Elements and Molecules
This PowerPoint presentation with worked examples and student questions covers:
• State the number of elements in a chemical formula.
• Determine the chemical formula from display formula.
• Dot and cross diagrams for bonded atoms

GCSE Chemistry: Covalent Structures
This PowerPoint presentation with worked examples and student questions covers:
• Definition of giant covalent structures
• An empirical formula shows the simplest whole-number ratio of the atoms of each compound.
• Melting and boiling point of simple molecules
• Compare physical properties of simple molecules and giant covalent lattices.

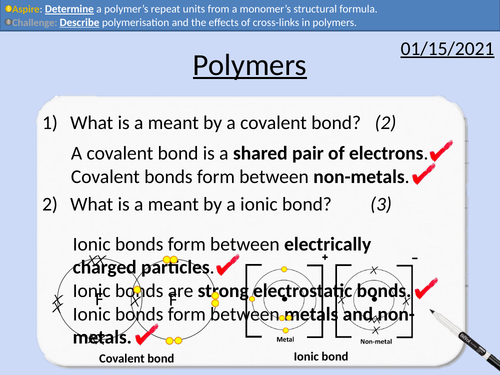
GCSE Chemistry: Polymers
This PowerPoint presentation with worked examples and student questions covers:
• State what is meant by mono- and poly-.
• Describe polymerisation and the effects of cross-links in polymers.
• Determine a polymer’s repeat units from a monomer’s structural formula.

OCR Applied Science: 21.2.3 Effectiveness of Tests
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.3 of Module 21: Product Testing Techniques.
2.3 Effectiveness of test
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use

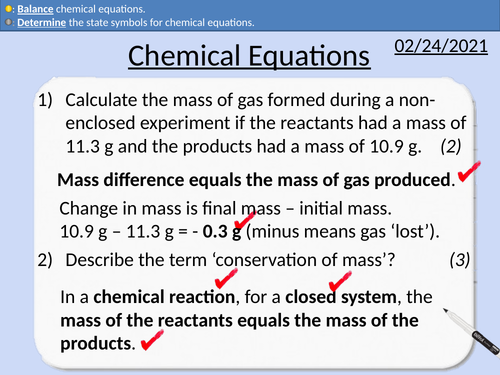
GCSE Chemistry: Chemical Equations
This PowerPoint presentation with worked examples and student questions covers:
• Pathways into medical chemistry
• State the number of atoms from a chemical formula.
• Properties of metals and non-metals
• Determine state symbols for chemical equations
• Balancing chemical equations

GCSE Chemistry: Conservation of Mass
This PowerPoint presentation with worked examples and student questions covers:
• State the number of atoms from a chemical formula.
• Relative Atomic masses and relative formula mass
• Practical activity of non-closed chemical reactions.

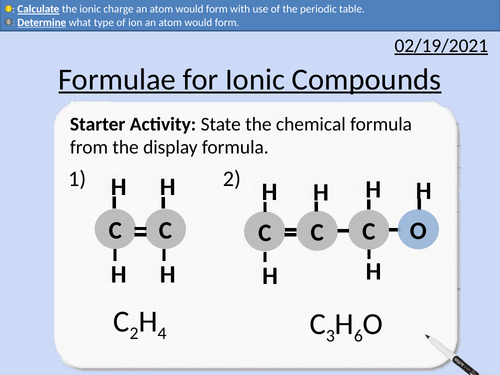
GCSE Chemistry: Formulae for Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• State the number of electrons in each energy level.
• Determine what type of ion an atom would form.
• Calculate the ionic charge an atom would form with use of the periodic table.
• Groups number, outer shell electrons, dot and cross diagrams
Bundle

GCSE OCR Chemistry C2 Elements, Compounds, and Mixtures
Resources for P2 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Relative Formula Mass
Empirical Formula
Pure and Impure Substances
Filtration and Crystallisation
Simple Distillation
Paper Chromatography
Purification and Checking Purity
Metals and Non-metals
Electronic Structures
Forming Ions
Ionic Compounds
Simple Molecules
Giant Covalent Structures
Polymer Molecules
Structure of Metals
Carbon
Changing State
Bulk Properties
Nanoparticles

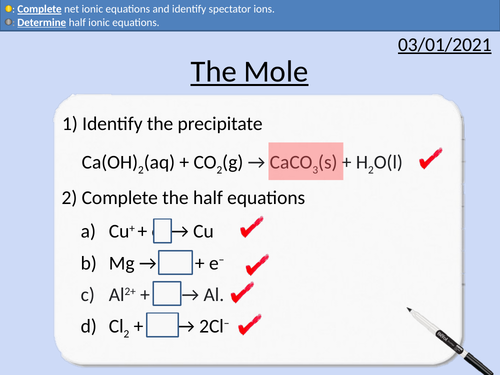
GCSE Chemistry: The Mole
This PowerPoint presentation with worked examples and student questions covers:
• Using Standard Form
• Avogadro’s constant
• Relative Atomic Mass, Relative Formula Mass and Molar Mass
• Rearranging Equations
• Calculating the number of moles present

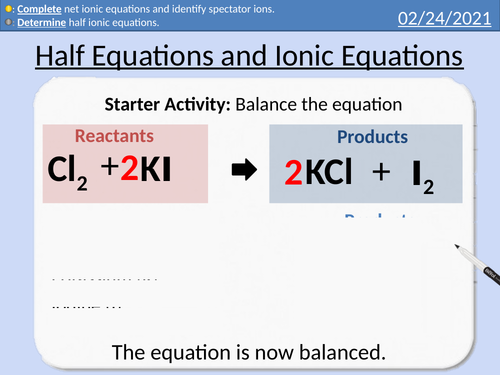
GCSE Chemistry: Half Equations and Ionic Equations
This PowerPoint presentation with worked examples and student questions covers:
• Precipitation in chemical reactions
• Definition of ions
• Ionic Half equations
• Dot and cross diagrams for electron structure
• Introduction to full ionic equations and net ionic equations