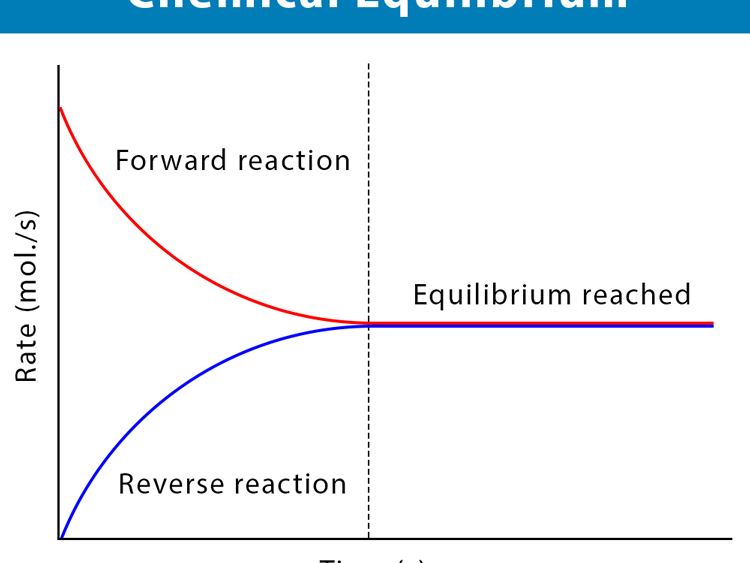

Dynamic equilibrium explained
Forward and reverse reactions
Closed systems
Rate of reaction equality at equilibrium
Le Chatelier’s Principle
Effect of concentration changes
Effect of pressure changes (gaseous systems)
Effect of temperature changes
Predicting direction of shift
Applying reasoning to unfamiliar reactions
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£5.00