
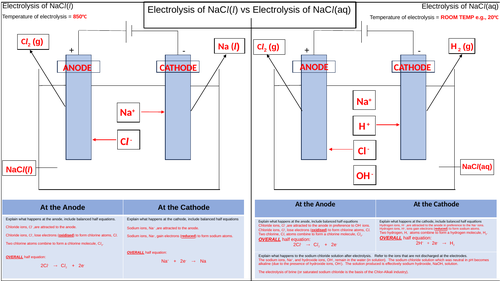
This is a pupil activity to visualise and summarise the electrolysis of molten and aqueous sodium chloride. The aims are to highlight the key differences between the two processes.
-
The temperatures of each electrolysis are stated to help emphasise one of the differences between the two processes. Sometimes pupils do not appreciate that the molten electrolysis must take place above the melting point of the ionic compound whereas aqueous electrolysis is usually performed at room temperature.
-
The cells as drawn and boxes provided for the ions present in each electrolyte so that again, a visual stimulus is used to show what ions are present in each electrolyte.
-
Arrows are drawn to shown which ions are attracted ti the appropriate electrodes with further arrows to show what products are formed at the anode and cathode for each process.
-
The boxes at the bottom are there so that the pupils can use correct key terms and phrases to explain in words what happens at each electrode, thereby helping to improve their literacy.
-
A teacher version is also provided to provide model answers for this activity.
This activity can be completed online or If possible, print this resource onto A3 size paper to allow pupils enough space to write answers clearly.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.