





This is an engaging revision lesson which uses a range of exam questions, understanding checks, quiz tasks and quiz competitions to enable students to assess their understanding of the content within topic 4 (Stoichiometry) of the Cambridge IGCSE Chemistry (0620) specification. The lesson covers the content in both the core and supplement sections of the specification and therefore can be used with students who will be taking the extended papers as well as the core papers.
The specification points that are covered in this revision lesson include:
CORE
- Use the symbols of the elements and write the formulae of simple compounds
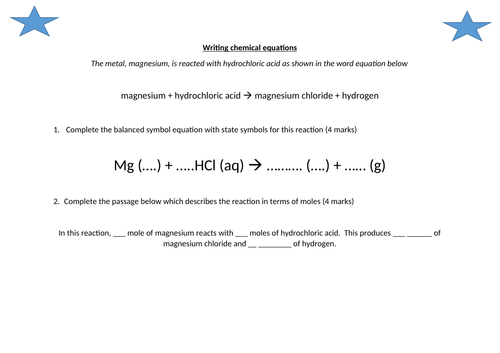
- Construct word equations and simple balanced chemical equations
- Define relative molecular mass, Mr, as the sum of the relative atomic masses
SUPPLEMENT
- Determine the formula of an ionic compound from the charges on the ions present
- Construct equations with state symbols
- Define the mole and the Avogadro constant
- Use the molar gas volume, taken as 24 dm3 at room temperature and pressure
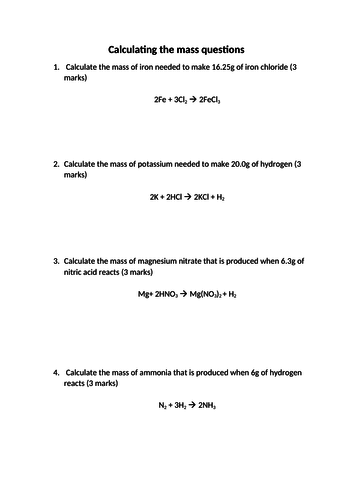
- Calculate stoichiometric reacting masses, volumes of gases and solutions, and concentrations of solutions expressed in mol / dm3.
The students will thoroughly enjoy the range of activities, which include quiz competitions such as “In the BALANCE” where they have to compete to be the 1st to balance an equation and recognise the number of moles involved whilst crucially being able to recognise the areas of this topic which need their further attention. This lesson can be used as revision resource at the end of the topic or in the lead up to mocks or the actual GCSE exams.
Get this resource as part of a bundle and save up to 50%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
Love it! Thanks - it's saved me a lot of time
Great, thank you! There's loads here. Also, thank you so much for keeping it free.
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.