



This is an engaging revision lesson which uses a range of exam questions, understanding checks, quick tasks and quiz competitions to enable students to assess their understanding of the content within topic 6 (Groups in the Periodic Table) of the Edexcel GCSE Chemistry specification.
The specification points that are covered in this revision lesson include:
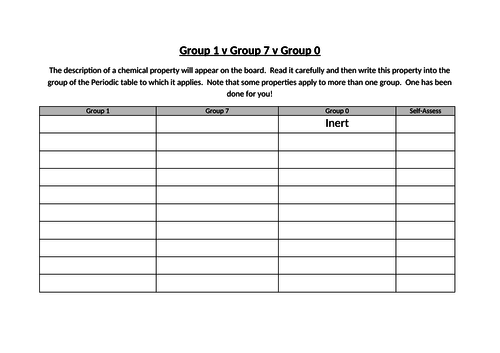
- Explain why some elements can be classified as alkali metals (group 1), halogens (group 7) or noble gases (group 0), based on their position in the periodic table
- Describe the pattern in reactivity of the alkali metals, lithium, sodium and potassium, with water; and use this pattern to predict the reactivity of other alkali metals
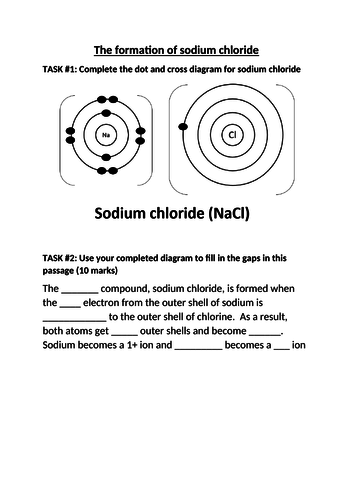
- Explain this pattern in reactivity in terms of electronic configurations
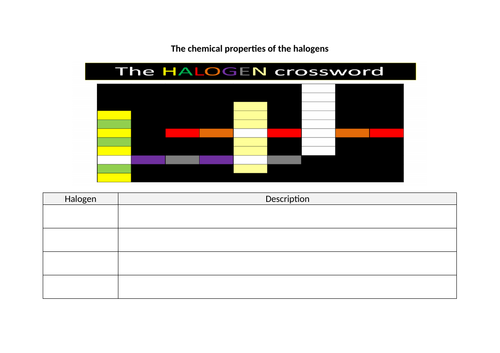
- Recall the colours and physical states of chlorine, bromine and iodine at room temperature
- Describe the pattern in the physical properties of the halogens, chlorine, bromine and iodine, and use this pattern to predict the physical properties of other halogens
- Describe the reactions of the halogens, chlorine, bromine and iodine, with metals to form metal halides, and use this pattern to predict the reactions of other halogens
- Describe the relative reactivity of the halogens chlorine, bromine and iodine, as shown by their displacement reactions with halide ions in aqueous solution, and use this pattern to predict the reactions of astatine
- Explain the relative reactivity of the halogens in terms of electronic configurations
- Explain why the noble gases are chemically inert, compared with the other elements, in terms of their electronic configurations
The students will thoroughly enjoy the range of activities, which include quiz competitions such as “Make sure you check every passage PERIODICALLY” where they have to scan summary passages about the table and decide if it is 100% correct whilst crucially being able to recognise the areas of this topic which need their further attention. This lesson can be used as revision resource at the end of the topic or in the lead up to mocks or the actual GCSE exams
Get this resource as part of a bundle and save up to 36%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Edexcel GCSE Chemistry REVISION LESSONS
This bundle of 7 revision lessons covers the content in the following topics of the Edexcel GCSE Chemistry specification Topic 1: Key concepts in Chemistry Topic 3: Chemical changes Topic 5: Separate chemistry 1 Topic 6: Groups in the Periodic Table Topic 7: Rates of reaction and energy changes Topic 8: Fuels and Earth Science Topic 9: Separate Chemistry 2 These lessons use a range of activities which include exam questions with fully explained answers, differentiated tasks and engaging quiz competitions to enable the students to assess their understanding of the different topics and crucially to recognise those areas which need further attention.
Edexcel GCSE Chemistry Paper 2 REVISION LESSONS
This bundle of 5 revision lessons covers the content which is found in Topics 1, 6, 7, 8 and 9 of the Edexcel GCSE Chemistry specification and therefore can be assessed on Paper 2 in the terminal exams. Topic 1: Key concepts in Chemistry Topic 6: Groups in the Periodic Table Topic 7: Rates of reaction and energy changes Topic 8: Fuels and Earth Science Topic 9: Separate Chemistry The lessons uses a range of activities which include exam questions with fully explained answers, differentiated tasks and engaging quiz competitions to enable the students to assess their understanding of the different topics and crucially to recognise those areas which need further attention.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.