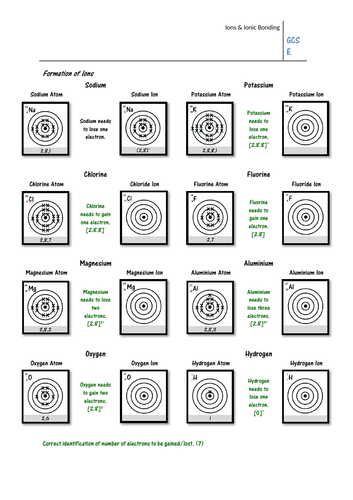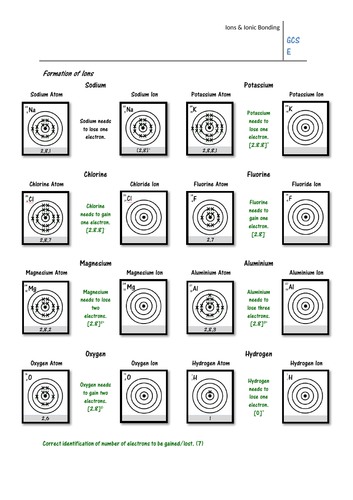
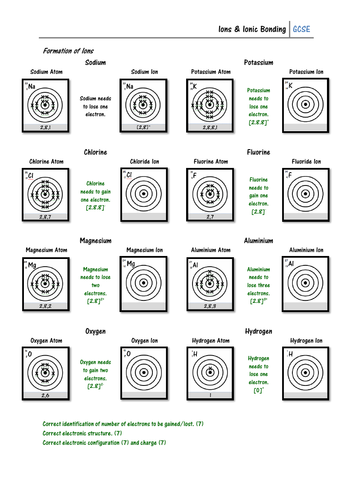
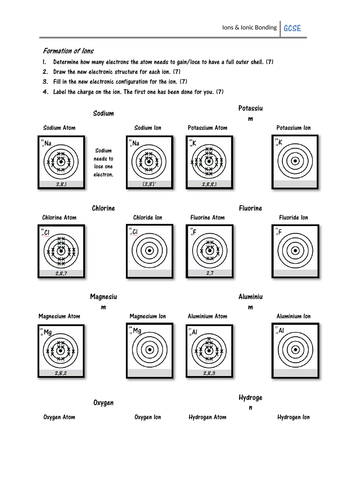
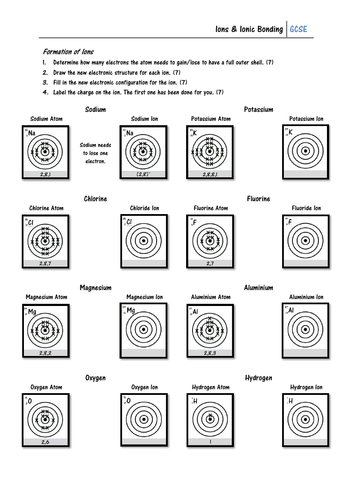
Pupils must look at the electron configuration of a neutral atom (e.g. sodium) and work out whether it will gain or lose electrons to form an ion. They must then draw the electron configuration of the ion, and give its charge. On the second page pupils show how ionic bonds can be formed using written electron configurations, and some may work out the formula of each ionic compound.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£2.00