Endothermic and Exothermic reactions
Keywords: Exothermic, endothermic, energy, temperature
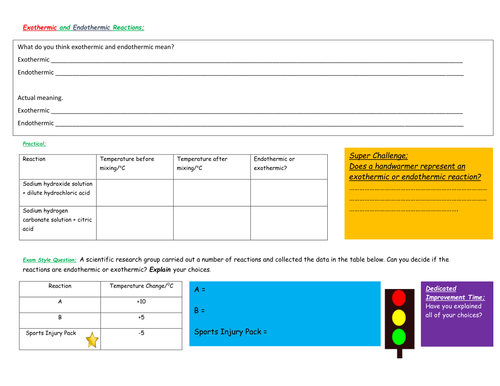
ALL: Define the terms exothermic and endothermic
MOST: interpret experimental data with respect to endothermic and exothermic reactions
SOME: describe examples of exothermic and endothermic reactions
S: Think, pair & share- what is the difference between exothermic and endothermic?
I: Introduce the idea of exothermic and endothermic reaction:
M:
A1- exothermic or endothermic reaction practical (10 min)
A2- Exothermic or Endothermic discussion in small groups (fire, self-heating cans and ice)
A3- Amber assistance/dedicated improvement time.
P- Exothermic or Endothermic
Stand up= Exothermic
Sit Down = Endothermic
Dilute solutions of sodium hydroxide, hydrochloric acid, sodium hydrogen carbonate and citric acid.
Keywords: Exothermic, endothermic, energy, temperature
ALL: Define the terms exothermic and endothermic
MOST: interpret experimental data with respect to endothermic and exothermic reactions
SOME: describe examples of exothermic and endothermic reactions
S: Think, pair & share- what is the difference between exothermic and endothermic?
I: Introduce the idea of exothermic and endothermic reaction:
M:
A1- exothermic or endothermic reaction practical (10 min)
A2- Exothermic or Endothermic discussion in small groups (fire, self-heating cans and ice)
A3- Amber assistance/dedicated improvement time.
P- Exothermic or Endothermic
Stand up= Exothermic
Sit Down = Endothermic
Dilute solutions of sodium hydroxide, hydrochloric acid, sodium hydrogen carbonate and citric acid.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00