13 Reversible reactions & Equilibrium
H/W before this lesson could be to research the work of Le Chatelier of the life of Fritz Haber (could lead to a discussion about Fritz Haber)
Hook/Starter: Can a reaction go backwards?
Pour the copper sulfate solution into the conical flask. Slowly add the acid down the side of the flask and swirl vigorously. Carefully add the ammonia in the same way but initially without swirling. Wait and show the students the colour change. Continue to add the ammonia with gentle swirling as the colour eventually changes to dark blue. Reverse the reaction by adding acid in a similar fashion to the ammonia.
I: What is a reversible reaction.
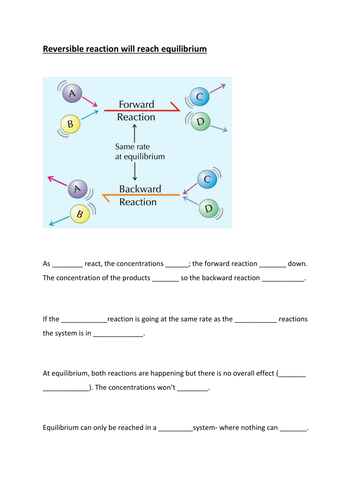
M: A1- Equilibrium. LA word fill. For HA print image on slide 5or6.
A2- position of equilibrium + introduce the idea of the factors which effect equilibrium
A3- Exothermic and Endothermic reversible reactions
A4- Show Haber process video which introduces the idea of Le Chatelier’s principle (with questions)
For HA could have a debate in class about Fritz Haber- should he have gotten a Nobel prize
P: Keyword Guru. In pairs, produce a list of key words and definitions which link to today’s lesson.
Then, jumble them up and challenge another pair to match the correct word to the correct definition. 2 L conical flask
100 mL 0.2 copper sulfate
250 mL concentrated ammonia solution
250 concentrated hydrochloric acid
Fume hood
Anhydrous and hydrous copper sulfate.
14 Le Chatelier’s principle and shifting equilibrium
Keywords: reversible, equilibrium, reactants, products, concentration, temperature and pressure
ALL: define Le Chatelier’s principle
MOST: describe the effect of changing concentration, temperature and pressure
SOME: interpret data and predict or suggest the best conditions
S: Watch Le Chatelier’s video part 1- What do pupil think Le Chatelier’s ideas are from this video?
I: define Le Chatelier’s principle (could model with cones from PE or coins)
Main
A1: recall the factor which effect equilibrium AfL and discuss in more detail (word fill for LA)
A2:Show video part 2 to recap
A3: Review questions (whiteboards)
P: Monster sentence challenge (reinforces key definitions for equilibrium) https://www.tes.com/teaching-resource/equilibrium-6025570 (FREE on TES)
H/W before this lesson could be to research the work of Le Chatelier of the life of Fritz Haber (could lead to a discussion about Fritz Haber)
Hook/Starter: Can a reaction go backwards?
Pour the copper sulfate solution into the conical flask. Slowly add the acid down the side of the flask and swirl vigorously. Carefully add the ammonia in the same way but initially without swirling. Wait and show the students the colour change. Continue to add the ammonia with gentle swirling as the colour eventually changes to dark blue. Reverse the reaction by adding acid in a similar fashion to the ammonia.
I: What is a reversible reaction.
M: A1- Equilibrium. LA word fill. For HA print image on slide 5or6.
A2- position of equilibrium + introduce the idea of the factors which effect equilibrium
A3- Exothermic and Endothermic reversible reactions
A4- Show Haber process video which introduces the idea of Le Chatelier’s principle (with questions)
For HA could have a debate in class about Fritz Haber- should he have gotten a Nobel prize
P: Keyword Guru. In pairs, produce a list of key words and definitions which link to today’s lesson.
Then, jumble them up and challenge another pair to match the correct word to the correct definition. 2 L conical flask
100 mL 0.2 copper sulfate
250 mL concentrated ammonia solution
250 concentrated hydrochloric acid
Fume hood
Anhydrous and hydrous copper sulfate.
14 Le Chatelier’s principle and shifting equilibrium
Keywords: reversible, equilibrium, reactants, products, concentration, temperature and pressure
ALL: define Le Chatelier’s principle
MOST: describe the effect of changing concentration, temperature and pressure
SOME: interpret data and predict or suggest the best conditions
S: Watch Le Chatelier’s video part 1- What do pupil think Le Chatelier’s ideas are from this video?
I: define Le Chatelier’s principle (could model with cones from PE or coins)
Main
A1: recall the factor which effect equilibrium AfL and discuss in more detail (word fill for LA)
A2:Show video part 2 to recap
A3: Review questions (whiteboards)
P: Monster sentence challenge (reinforces key definitions for equilibrium) https://www.tes.com/teaching-resource/equilibrium-6025570 (FREE on TES)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£2.00