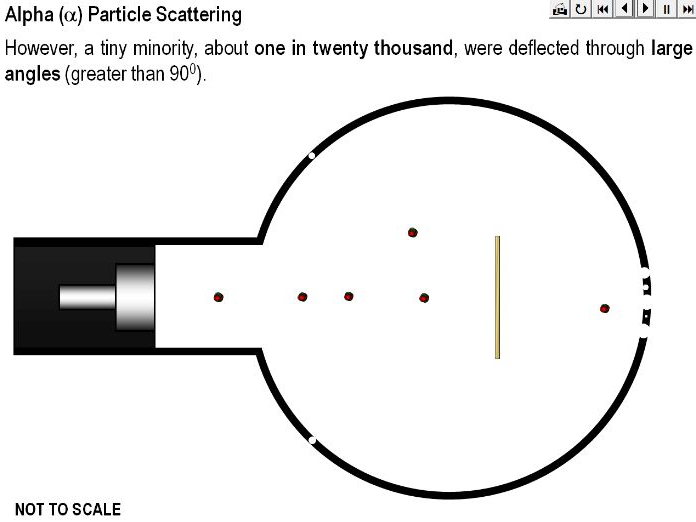



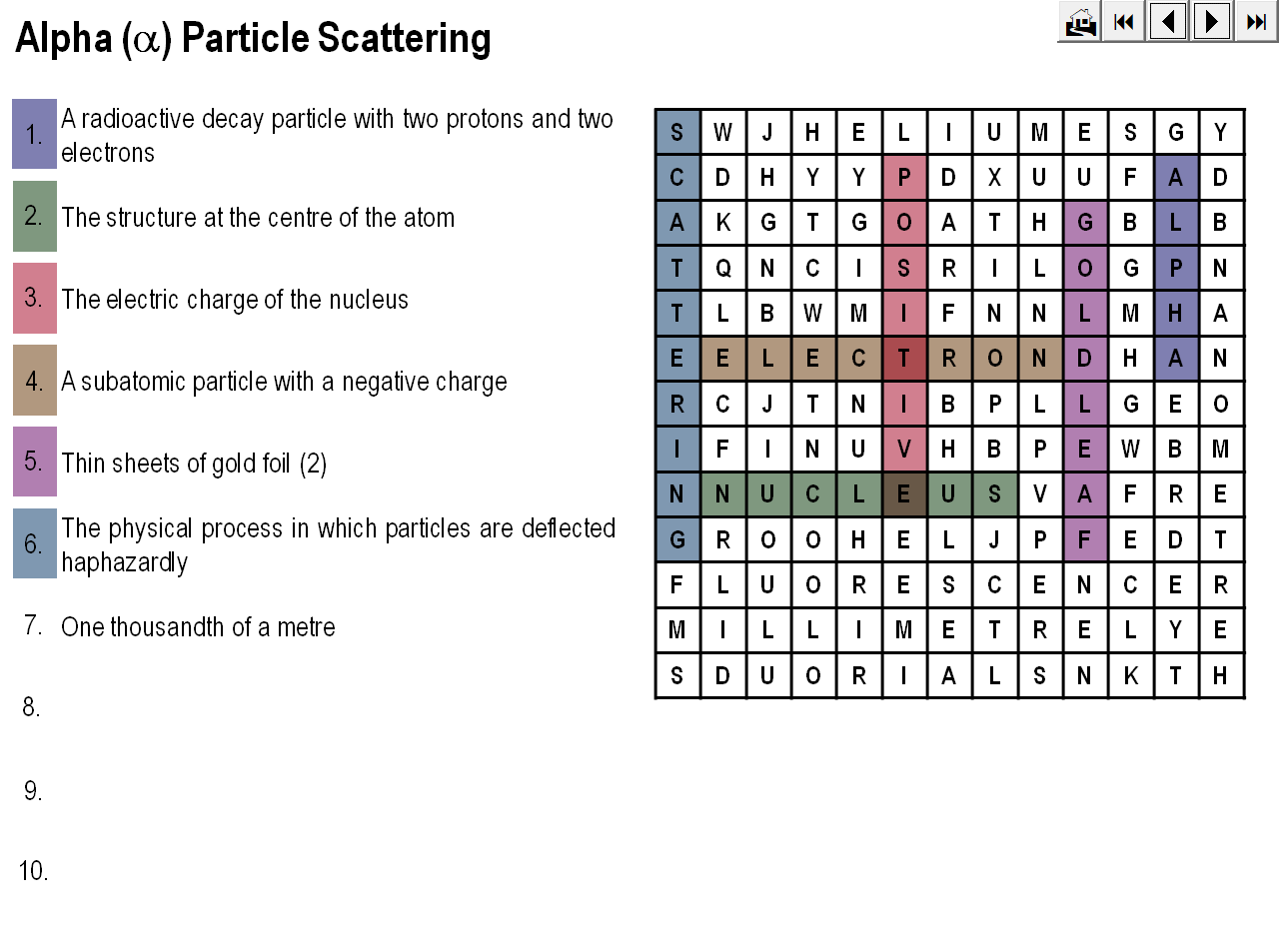
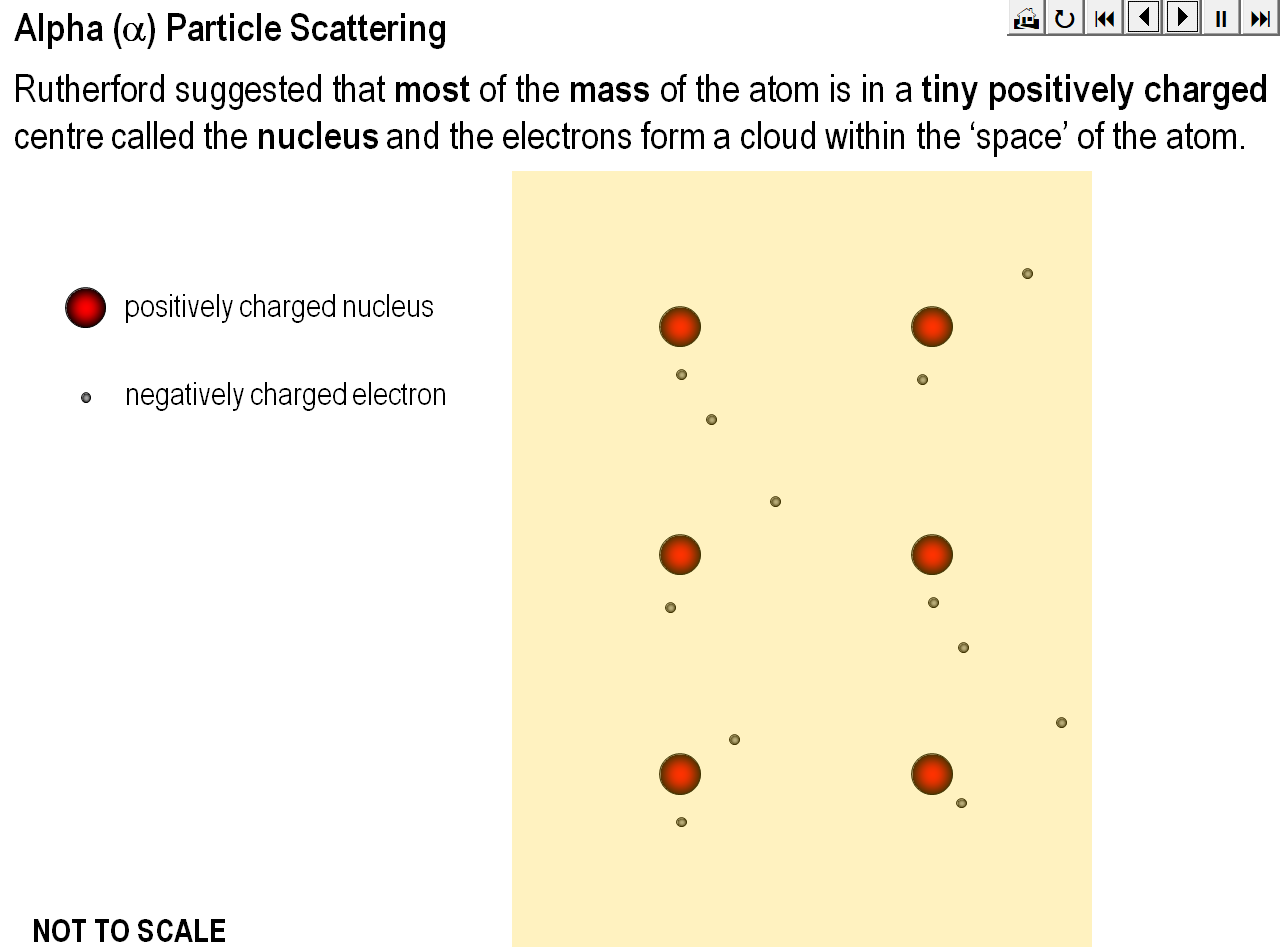






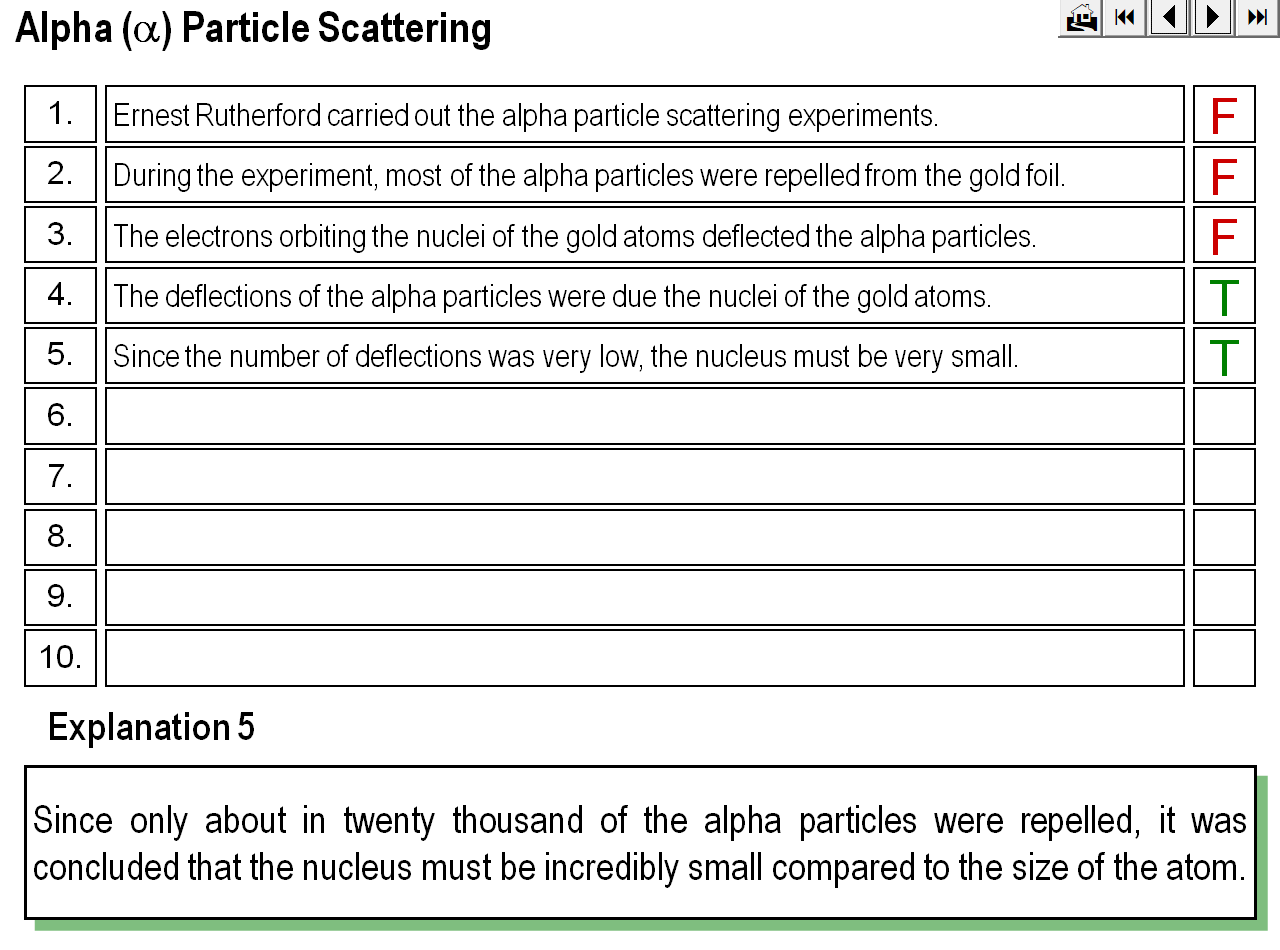
An animation demonstrating how Rutherford’s alpha particle scattering experiment showed that the mass of an atom is concentrated within a space that is less than 99.99% of the total volume of the atom.
I have many others of the same format; these can be seen by visiting my shop on the TES website (search: rtyler62).
If you buy this resource, please print the Readme document as it contains the instructions and details of the files included.
NOTE: MACROS MUST BE ENABLED FOR ALL POWERPOINT PRESENTATIONS
Support Material
- Readme (instructions for whole lesson)
- Learning outcomes (PowerPoint)
- Starter activity (PowerPoint and worksheet)
- Main activity (PowerPoint presentation)
- Assessment (worksheets with answer sheets - differentiated)
- Lesson notes (handout – 2 x A5 on A4 paper)
- Plenary activity (PowerPoint and worksheet)
Users and Timings
It is intended for physics teachers and is aimed at pupils over 16 years old. Normally, this resource would fill a 45 to 60 minute lesson but could easily be extended to two lessons.
Learning Outcomes
The learning outcomes are based on Bloom’s taxonomy of hierarchical classification: knowledge, comprehension, application, analysis, synthesis and evaluation. The lesson title and learning outcomes are:
How can the structure of an atom be determined if it can’t be seen?
Learning outcomes
Knowledge
To recount Rutherford’s alpha particle scattering experiment.
Comprehension
To explain why this experiment led to a better understanding of the structure of the atom.
Differentiation:
The activities have varying degrees of differentiation; please refer to the Readme document.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.