AQA GCSE Combined Science - **FOUNDATION TIER **
These simple revision mats can be printed A3 with a single sheet forming a revision activity for an entire topic. Include a mixture of cloze word fact sections with recall and exam style questions. Now with free suggested answer sheets to support non-specialists or parents/students revising. Provided as Powerpoint and PDF.
These 12 printable A3 mats cover the following content
Unit 3 Quantitative Chemistry (3 mats)
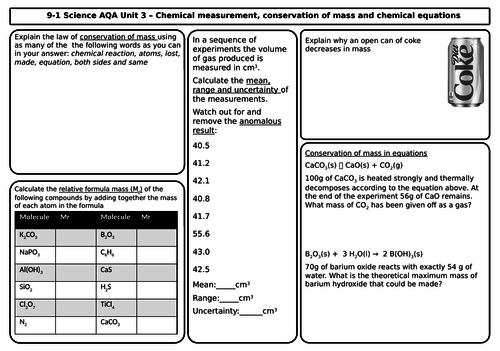
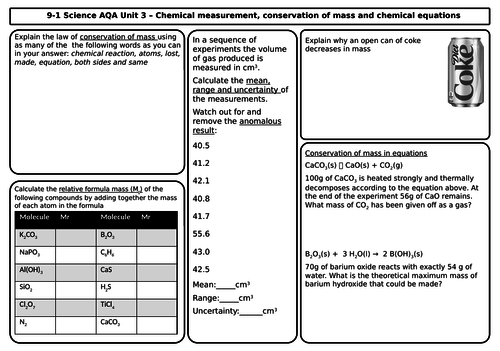
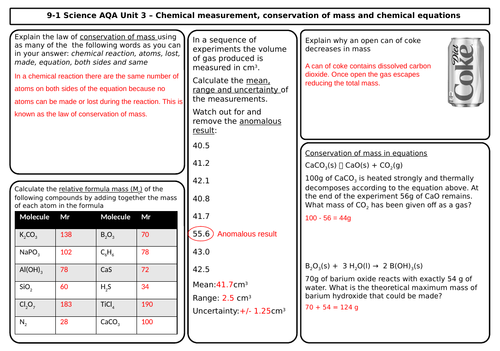
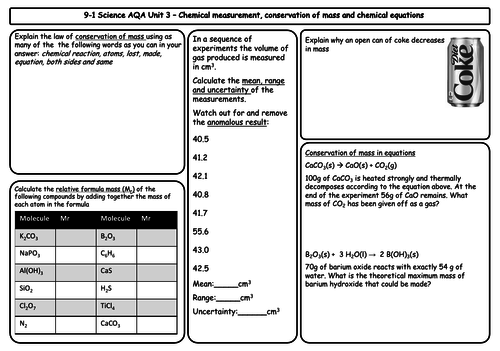
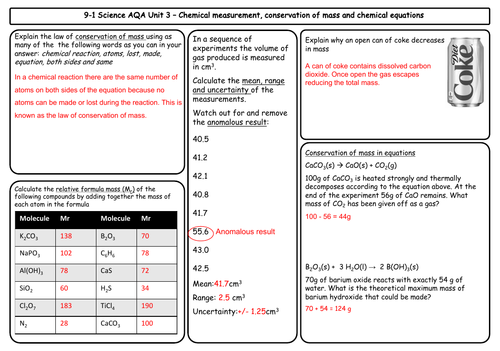
- Conservation of mass and balanced chemical equations
- Relative formula mass
- Mass changes when a reactant or product is a gas
- Chemical measurements
- Concentrations of solutions
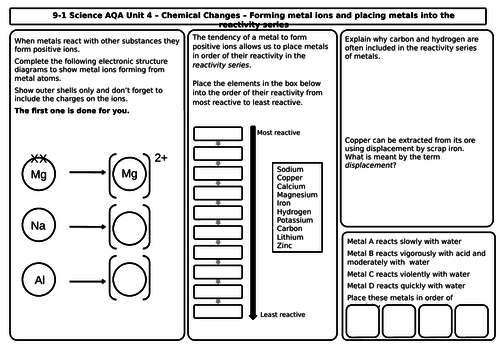
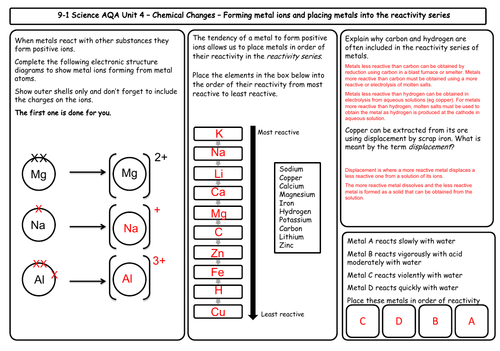
Unit 4 Chemical Changes (9 mats)
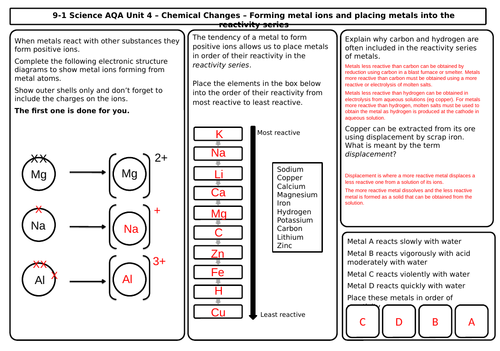
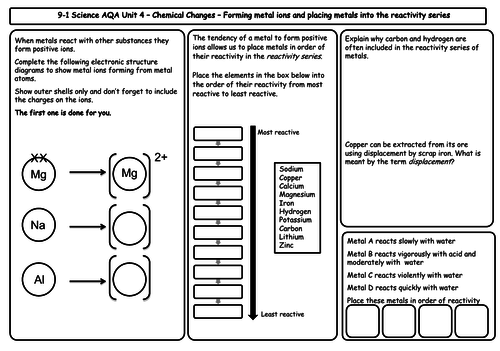
- The reactivity of metals
- The reactivity series
- Extraction of metals and reduction
- Reactions of acids with metals
- Neutralisation of acids and salt production
- Soluble salts
- The pH scale and neutralisation
- Electrolysis of molten ionic compounds
- Electrolysis in aqueous solution
- Using electrolysis to extract metals
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.