
Edexcel IGCSE Chemistry | Unit 3.16 | Physical Chemistry | Required Practical
This Synapse Science resource supports Required Practical 3.16 from the Edexcel IGCSE Chemistry specification. Students investigate how different solid catalysts affect the rate of decomposition of hydrogen peroxide and record the volume of oxygen gas produced over time.
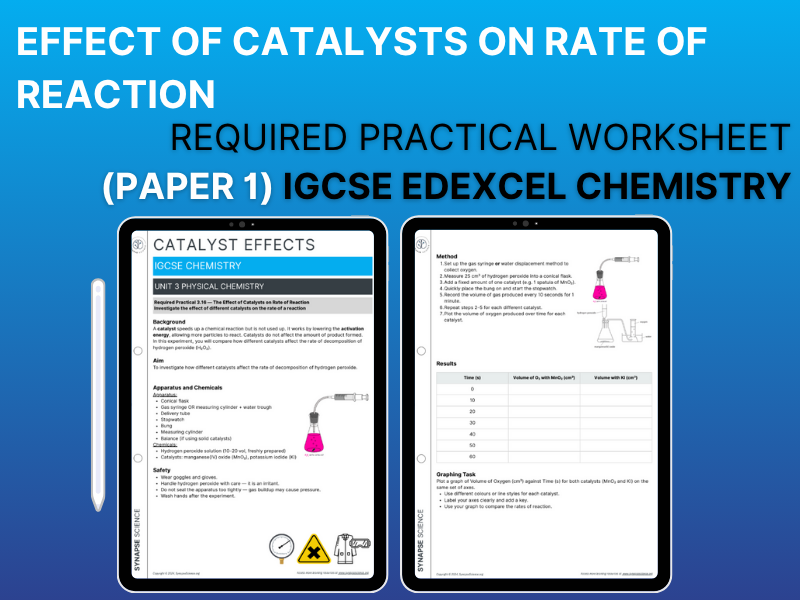
Specification Point Covered- 3.16: Practical – Investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide solution
-
Student Worksheet –
• Method using MnO₂ and KI
• Results table with time vs gas volume
• Graphing task: compare reaction rates on one set of axes
• Scaffolded conclusion with success criteria -
Teacher & Technician Notes –
• Setup overview and safety guidance
• Expected results and model conclusion
• MSDS summary for hydrogen peroxide and catalysts
- Simple, effective classroom practical using common catalysts
- Visual comparison of reaction rates through graphing task
- Emphasises catalyst function (faster reaction, not used up)
- Ideal for reinforcing activation energy and reaction kinetics
- Clean Synapse Science format – ready for print or digital use
Edexcel IGCSE Chemistry, Required Practical, Catalysts, Manganese Dioxide, Potassium Iodide, Hydrogen Peroxide, Rate of Reaction, Oxygen Volume, Activation Energy, Physical Chemistry, Synapse Science, Worksheet, Graphing Task
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.