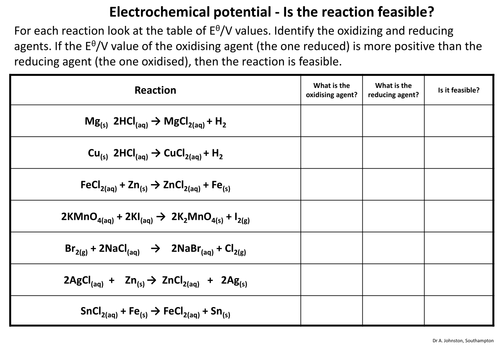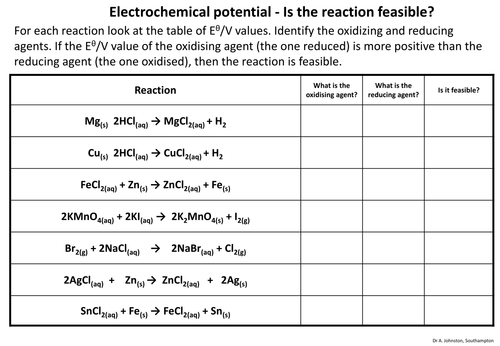
This is an activity where the students have to identify the oxidizing and reducing agents in a redox reaction, then say whether the reaction is feasible based on the electrochemical potentials of the reactants.
Something went wrong, please try again later.
This is an excellent introduction to cell potentials and redox feasibility, with minor correction needed. The fourth reaction shows Manganate(VII) being reduced to Manganate(VI), which has a half cell potential of +0.56V. This ionic half equation is not given in the data. The manganate(VII) to manganese(II) does not apply in this case. Apologies for putting this in a comment. I'd rather have contacted the author directly but couldn't see how.
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£0.00