



Get this resource as part of a bundle and save up to 20%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Fossil fuels lessons GCSE - outstanding lessons that provide support and challenge, recap prior learning, demonstrate progress
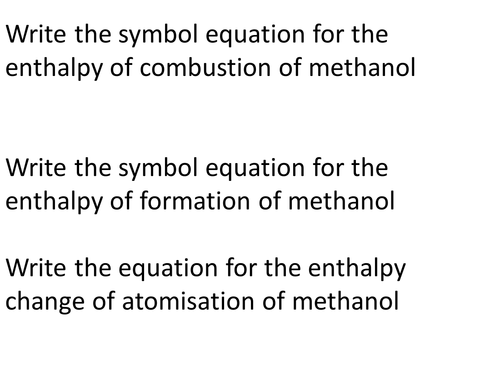
This is a set of 10 lessons including a revision lesson on fossil fuels. Included are lessons on combustion, formation of crude oil, fractional distillation, fermentation, alkanes, alkenes, calorimetry, bond enthalpy (advanced lesson for more able students) and alternatives to crude oil. Please take the opportunity to look at each of these lessons to find out more information and comment on them.
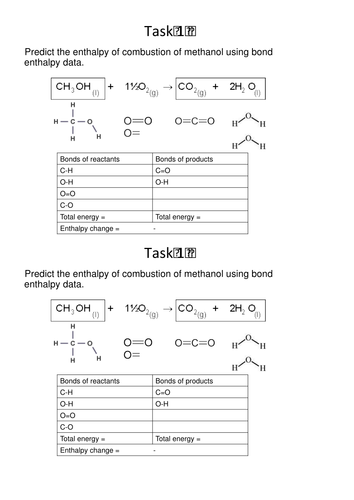
Enthalpy changes - includes Q=mc delta T, types of change, bond enthalpies
This is a bundle of resources for A level chemistry
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.