



**This Powerpoint specifically uses the Cambridge iGCSE Chemistry Syllabus and covers all criteria for – Unit 4.1 Electrolysis
**
Students will be able to -
1 Define electrolysis as the decomposition of an
ionic compound, when molten or in aqueous
solution, by the passage of an electric current
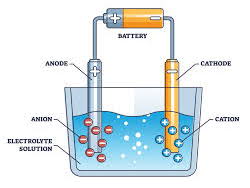
2 Identify in simple electrolytic cells:
(a) the anode as the positive electrode
(b) the cathode as the negative electrode
© the electrolyte as the molten or aqueous
substance that undergoes electrolysis
3 Identify the products formed at the electrodes
and describe the observations made during the
electrolysis of:
(a) molten lead(II) bromide
(b) concentrated aqueous sodium chloride
© dilute sulfuric acid
using inert electrodes made of platinum or
carbon/graphite
4 State that metals or hydrogen are formed at
the cathode and that non-metals (other than
hydrogen) are formed at the anode
5 Predict the identity of the products at each
electrode for the electrolysis of a binary
compound in the molten state
6 State that metal objects are electroplated to
improve their appearance and resistance to
corrosion
7 Describe how metals are electroplated
8 Describe the transfer of charge during
electrolysis to include:
(a) the movement of electrons in the external
circuit
(b) the loss or gain of electrons at the
electrodes
© the movement of ions in the electrolyte
9 Identify the products formed at the electrodes
and describe the observations made during the
electrolysis of aqueous copper(II) sulfate using
inert carbon/graphite electrodes and when
using copper electrodes
10 Predict the identity of the products at each
electrode for the electrolysis of a halide
compound in dilute or concentrated aqueous
solution
11 Construct ionic half-equations for reactions
at the anode (to show oxidation) and at the
cathode (to show reduction)
**Every Powerpoint includes learning objectives, a starter and plenary, suitable activities and review questions with mark scheme. We also provide a FREE pdf version of the file so it can easily be printed for students as a handout or uploaded to the school VLE system
**
Bring your Cambridge iGCSE Chemistry lessons to life with our expertly designed teaching resources. Each unit is tailored to the latest syllabus, offering ready-to-use lesson plans, engaging experiments, and visually clear notes that save hours of prep time. Whether you’re new to the course or refining your approach, The Science Shelf gives you everything you need to boost student understanding and exam success — all in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.