
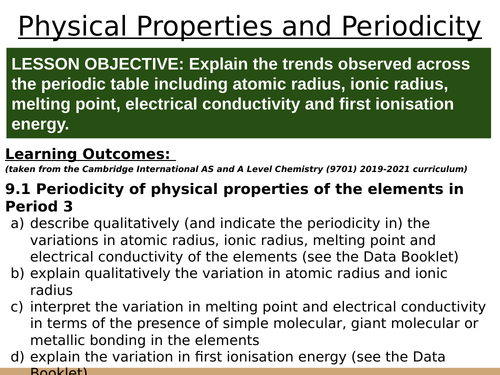
LESSON OBJECTIVE: Explain the trends observed across the periodic table including atomic radius, ionic radius, melting point, electrical conductivity and first ionisation energy.
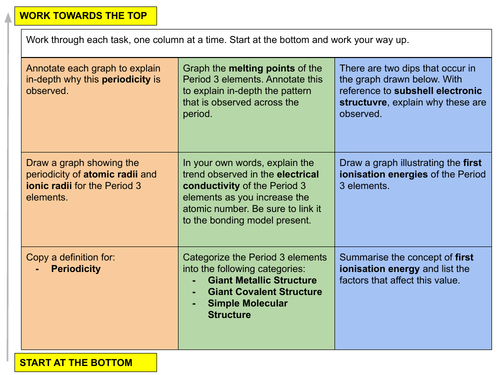
In this lesson we discuss the concept of periodicity and justify the trends we observe in a number of physical properties as we move across the Period 3 elements. This is lesson one in our inorganic chemistry series for Unit 9: The Periodic Table: chemical periodicity (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
Learning Outcomes:
(taken from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum)
9.1 Periodicity of physical properties of the elements in Period 3
a) describe qualitatively (and indicate the periodicity in) the variations in atomic radius, ionic radius, melting point and electrical conductivity of the elements (see the Data Booklet)
b) explain qualitatively the variation in atomic radius and ionic radius
c) interpret the variation in melting point and electrical conductivity in terms of the presence of simple molecular, giant molecular or metallic bonding in the elements
d) explain the variation in first ionisation energy (see the Data Booklet)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.