
LESSON OBJECTIVE: Understand the mechanism of SN1 and SN2 nucleophilic substitution reactions of halogenoalkanes and describe the relative strength of the C-Hal bond.
In this lesson we investigate the differences in reaction mechanism between SN1 and SN2 reactions, describe the factors that effect which one will be observed in a nucleophilic substitution reaction and investigate how the carbon-halogen bond strength effect the rate of reaction. This is lesson nine in our organic chemistry series of Unit 16: Halogen derivatives (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
Learning Outcomes:
(taken from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum)
16.1 Halogenoalkanes
a) recall the chemistry of halogenoalkanes as exemplified by:
(i) the following nucleophilic substitution reactions of bromoethane: hydrolysis, formation of nitriles, formation of primary amines by reaction with ammonia
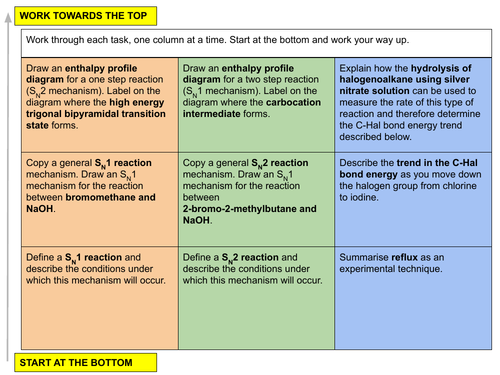
b) describe the SN1 and SN2 mechanisms of nucleophilic substitution in halogenoalkanes including the inductive effects of alkyl groups
c) recall that primary halogenoalkanes tend to react via the SN2 mechanism; tertiary halogenoalkanes via the SN1 mechanism; and secondary halogenoalkanes by a mixture of the two, depending on structure.
16.2 Relative strength of the C-Hal bond
a) interpret the different reactivities of halogenoalkanes (with particular reference to hydrolysis and to the relative strengths of the C-Hal bonds)
Something went wrong, please try again later.
Very concise but cover all the point <br /> I used it for my A2 Kinetic topic
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.