
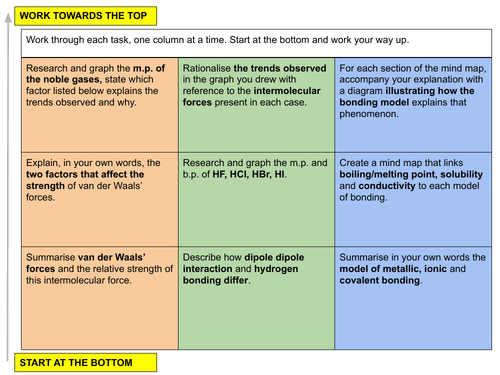
In this lesson we discuss the relative strength of intermolecular forces and how different types of chemical bonding will affect a species physical properties. This is lesson eleven in our physical chemistry series for Unit 3: Chemical Bonding (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
LESSON OBJECTIVE: Identify and rationalise the types of intermolecular forces a molecule will have and consequently describe and predict the physical properties of different species based on the type of bonding present.
Learning Outcomes (from the Cambridge AS Chemistry Curriculum 2019-2021):
3.5 Bonding and physical properties
a) describe, interpret and predict the effect of different types of bonding (ionic bonding, covalent bonding, hydrogen bonding, other intermolecular interactions, metallic bonding) on the physical properties of substances
b) deduce the type of bonding present from given information
c) show understanding of chemical reactions in terms of energy transfers associated with the breaking and making of chemical bonds
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.