
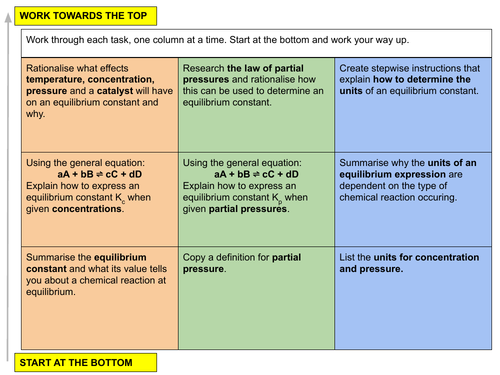
In this lesson we discuss equilibrium constants and how to determine them using concentrations and partial pressures, and discuss how certain factors can change the value of an equilibrium constant. This is lesson twenty one in our physical chemistry series for Unit 7: Equilibria (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
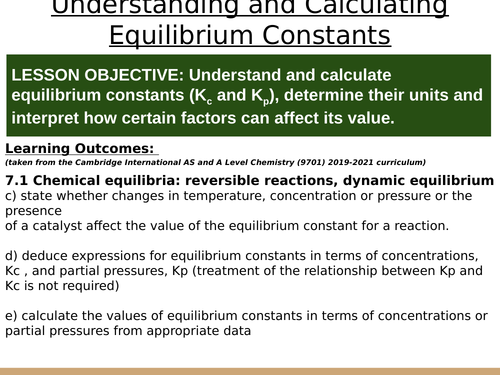
LESSON OBJECTIVE: Understand and calculate equilibrium constants (Kc and Kp), determine their units and interpret how certain factors can affect its value.
LEARNING OUTCOMES (taken from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum):
c) state whether changes in temperature, concentration or pressure or the presence
of a catalyst affect the value of the equilibrium constant for a reaction.
d) deduce expressions for equilibrium constants in terms of concentrations, Kc , and partial pressures, Kp (treatment of the relationship between Kp and Kc is not required)
e) calculate the values of equilibrium constants in terms of concentrations or partial pressures from appropriate data
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.