
In this lesson we describe the relationship between temperature and reaction rates and introduce the concept of the Boltzmann distribution. This is lesson twenty five in our physical chemistry series for Unit 8: Reaction kinetics (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
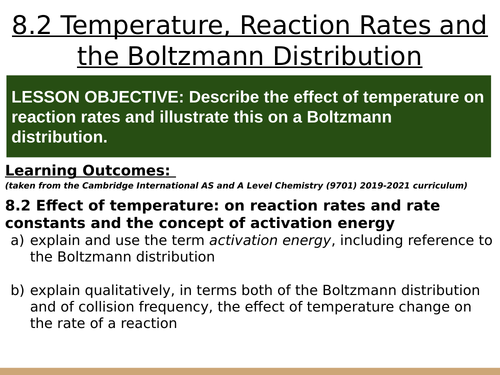
LESSON OBJECTIVE: Describe the effect of temperature on reaction rates and illustrate this on a Boltzmann distribution.
LEARNING OUTCOMES (taken from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum):
8.2 Effect of temperature: on reaction rates and rate constants and the concept of activation energy
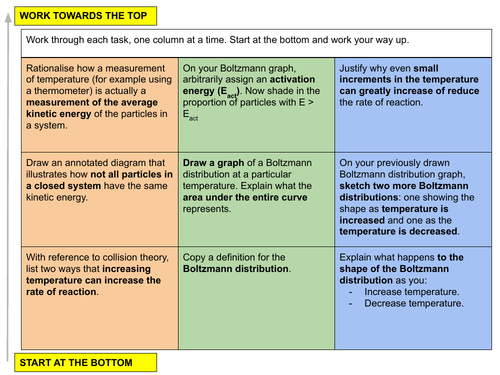
a) explain and use the term activation energy, including reference to the Boltzmann distribution
b) explain qualitatively, in terms both of the Boltzmann distribution and of collision frequency, the effect of temperature change on the rate of a reaction
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.