
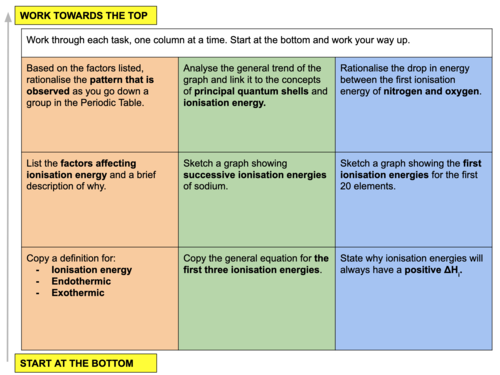
In this lesson we focus on the concept of ionisation energy and how to interpret ionisation energy trends in the periodic table. This is lesson seven in our physical chemistry series for Unit 2: Atomic Structure (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
LESSON OBJECTIVE: Understand ionisation energy and use this to rationalise trends in the Periodic Table and to deduce electronic configurations of elements. To Interpret ionisation energy data.
Learning Outcomes (from the Cambridge AS Chemistry Curriculum 2019-2021):
2.3 Electrons: energy levels, atomic orbitals, ionisation energy, electron
affinity
d) i) explain and use the term ionisation energy
ii) explain the factors influencing the ionisation energies of elements
iii) explain the trends in ionisation energies across a period and down a group of the Periodic Table
e) deduce the electronic configurations of elements from successive ionisation energy data
f) interpret successive ionisation energy data of an element in terms of the position of that element within the Periodic Table
Something went wrong, please try again later.
Really nice resource thanks so much. Great for taking from no knowledge to coping with questions!
Fabulous resource demonstrating excellent online delivery which I can reproduce
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.