
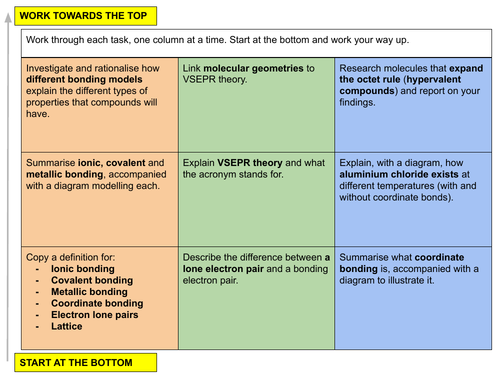
In this lesson we give an overview of the three types of chemical bonding (ionic, covalent and metallic) and an introduction into how VSEPR theory dictates molecular geometries. This is lesson eight in our physical chemistry series for Unit 3: Chemical Bonding (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
LESSON OBJECTIVE: Understand how ionic, covalent and metallic bonds form. Rationalise molecular geometries using VSEPR theory.
Learning Outcomes (from the Cambridge AS Chemistry Curriculum 2019-2021):
3.1 Ionic bonding
a) describe ionic bonding, using the examples of sodium chloride, magnesium oxide and calcium fluoride, including the use of ‘dot-and- cross’ diagrams
3.2 Covalent bonding and co-ordinate (dative covalent) bonding including shapes of simple molecules
a) describe, including the use of ‘dot-and-cross’ diagrams:
(i) covalent bonding, in molecules such as hydrogen, oxygen, chlorine, hydrogen chloride, carbon dioxide, methane, ethene
(ii) co-ordinate (dative covalent) bonding, such as in the formation of the ammonium ion and in the Al2Cl6 molecule
c) explain the shapes of, and bond angles in, molecules by using the qualitative model of electron-pair repulsion (including lone pairs), using as simple examples BF3 (trigonal planar), CO2 (linear), CH4 (tetrahedral), NH3 (pyramidal), H2O (non-linear), SF6 (octahedral), PF5 (trigonal bipyramidal)
3.4 Metallic bonding
a) describe metallic bonding in terms of positive ions surrounded by delocalised electrons
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.