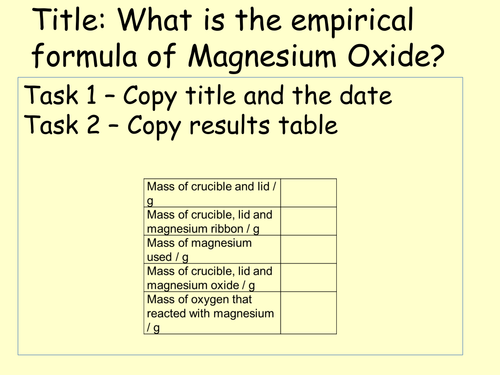
In this lesson students apply their knowledge of calculating empirical formula by reacting known masses of magnesium with unknown masses of oxygen to make a known mass of magnesium oxide which they can then use along with relative atomic masses to calculate the ratio of oxygen to magnesium and in turn the empirical formula of magnesium oxide.
Save yourself some time and grab a bargain!
Save yourself some time and grab a bargain!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£2.00