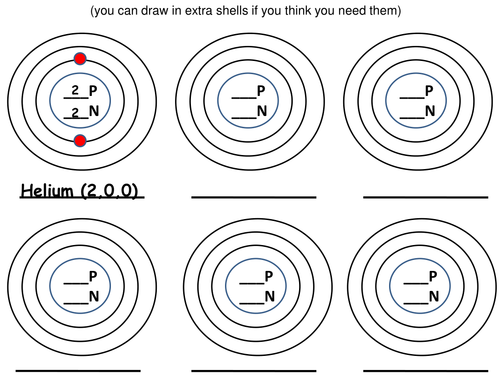
The second lesson in 'The Fundamental Ideas in Chemistry' topic looking at how electrons are arranged in shells.
Lesson objectives:
-Understand that elements in the same group in the periodic table have the same number of electrons in their highest energy level (outer electrons) and this gives them similar chemical properties.
-Know that elements in Group 0 of the periodic table are called the noble gases. They are unreactive because their atoms have stable arrangements of electrons.
Resources included:
x1 Powerpoint starter on identification of element from atomic number.
x1 PowerPoint with lesson objectives from AQA with main content of lesson and plenary.
x 4 worksheets, all based on understanding electron arrangement, chose which one depending on your students.
x2 teaching aid/animation good to use with interactive whiteboard or mouse (.swf files need Adobe Flash Player)
Lesson objectives:
-Understand that elements in the same group in the periodic table have the same number of electrons in their highest energy level (outer electrons) and this gives them similar chemical properties.
-Know that elements in Group 0 of the periodic table are called the noble gases. They are unreactive because their atoms have stable arrangements of electrons.
Resources included:
x1 Powerpoint starter on identification of element from atomic number.
x1 PowerPoint with lesson objectives from AQA with main content of lesson and plenary.
x 4 worksheets, all based on understanding electron arrangement, chose which one depending on your students.
x2 teaching aid/animation good to use with interactive whiteboard or mouse (.swf files need Adobe Flash Player)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00