Shapes of ions and molecules lesson for year 12 or year 13 (AS level or A level).
LOs:
To state the basic features of a molecule that determine its shape.
To describe different molecular structures.
To predict the shapes of simple molecules and ions with up to six electron pairs surrounding the central atom.
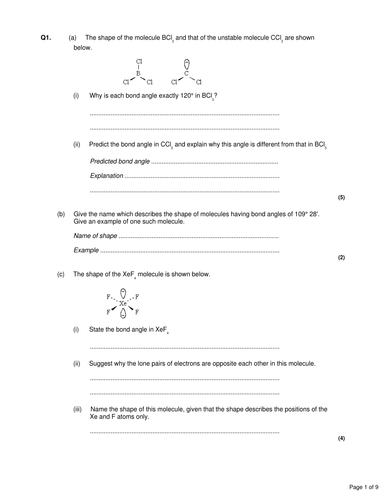
1. Do Now: Draw the Lewis structures for water, methane and ammonia. How many pairs of electrons surround the central atom in each case?
2. Exposition: VSEPR theory and structures with 4 areas of electron density
3. Mini-pleanry: Determine the shape of molecules that have 4 areas of electron density
4. Exposition: Shapes from 2 or 3 areas of electron density
5. Mini-plenary: Determine the shape of molecules that have 2, 3 or 4 areas of electron density
6. Exposition: Shapes with 5 or 6 areas of electron density
7. Exposition: How lone pairs affect bond angles
8. Independent Task: Practise determining shapes and bond angles of various different ions and molecules
7. Independent Task: Exam style questions on bond angles and molecular shape
8. Plenary: Wrapping it up
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.