This bundle covers all of the OCR A level chemistry specifications section 4.2 (alcohols, haloalkanes and analysis)
The resources included are:
- chemistry of alcohols
- haloalkanes
- organic synthesis
- AS synthetic routes
- infrared spectroscopy
- mass spectrometry
- identifying compounds from infrared and mass spectra
Most of the resources include a fully interactive PowerPoint including starter, group activities, questions and plenary along with a worksheet. Many of the PowerPoint slides contain links to other slides, to enable easy navigation and to emphasise links between different aspects. Some of the resources include a PowerPoint quiz and all are ideal for classroom or home learning.
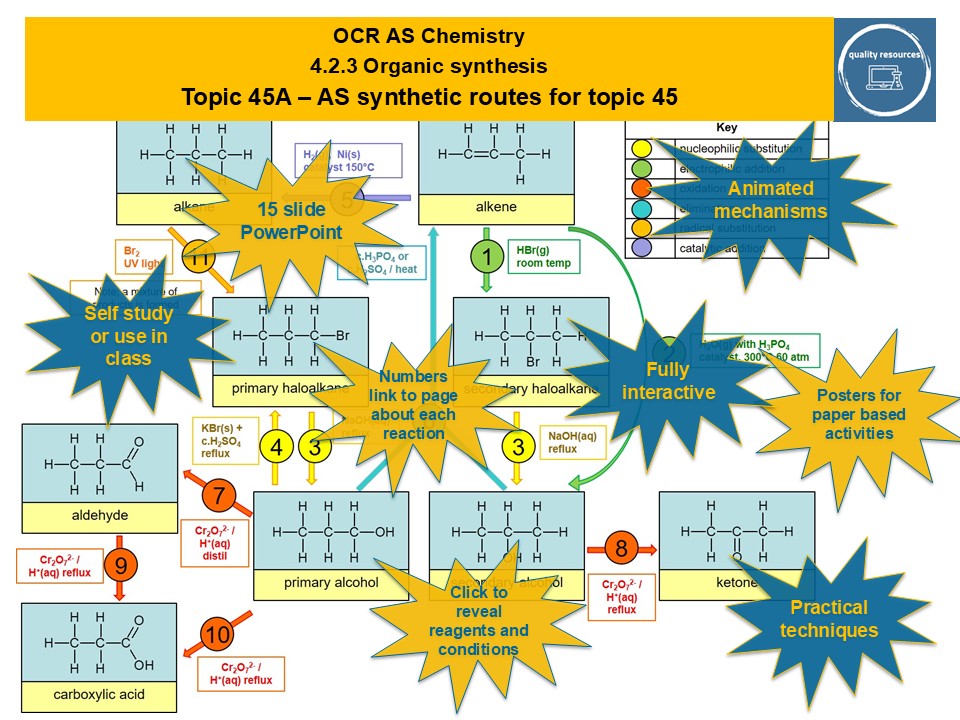
The synthetic routes resource covers AS organic synthetic routes through a 15 slide interactive PowerPoint that is based on a clear chart of numbered synthetic routes, where each number is linked to a page detailing that reaction. Information given includes type of reaction, reagents and conditions and an equation, as well as key definitions. In addition most reactions have an animated mechanism or structural equation. There are links to pages describing and explaining practical techniques, where relevant.
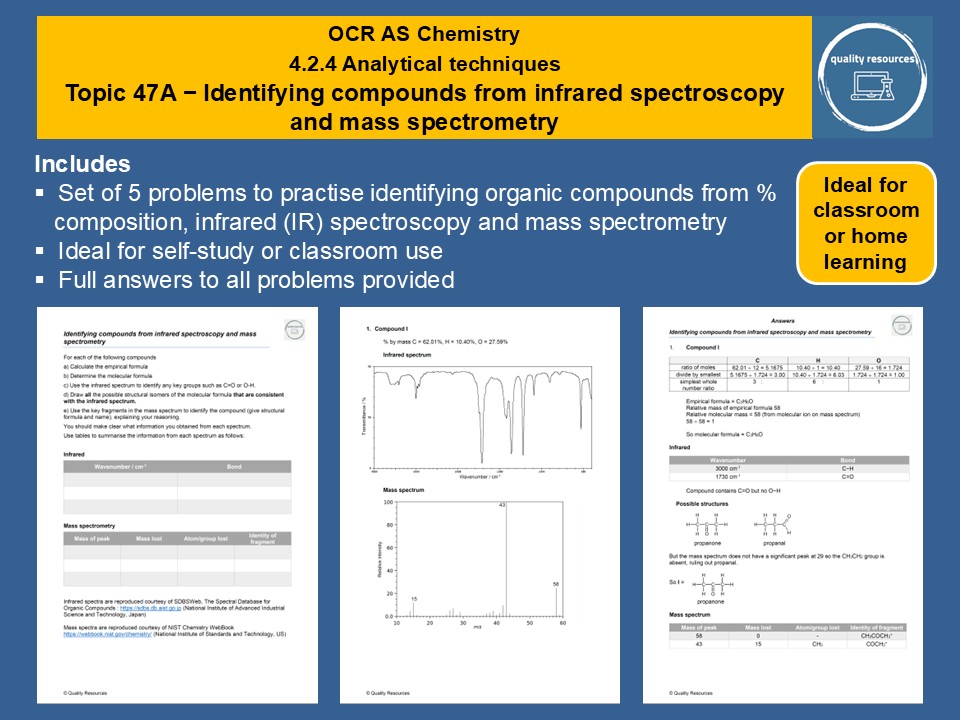
Also included is a resource consisting of a set of five problems to practise identifying organic compounds from % composition, infrared (IR) spectroscopy and mass spectrometry.
Full answers to all exercises are provided.
This bundle is part of a series covering the OCR AS Chemistry specification and relates to the following sections:
Module 4– Core organic chemistry
Part 2 –Alcohols, haloalkanes and analysis (all)
Please review!
Content covered:
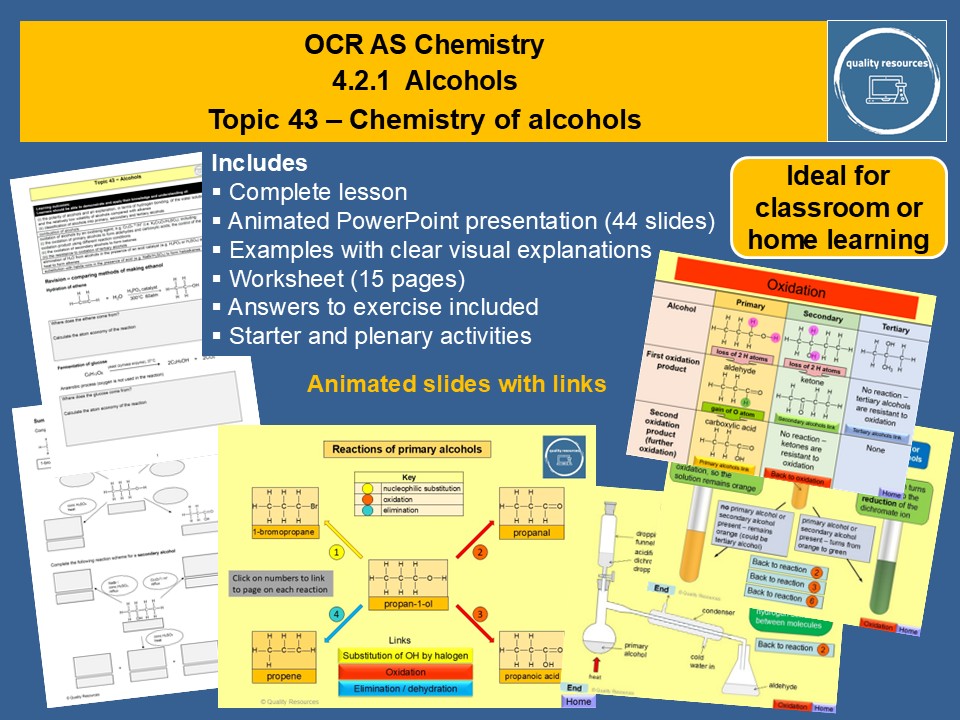
Chemistry of alcohols
• Comparing methods of making ethanol
• Naming alcohols
• Physical properties of alcohols, in terms of hydrogen bonding
• Primary, secondary and tertiary alcohols
• Substitution reaction of alcohols
• Oxidation of alcohols
• Elimination (dehydration) reaction of alcohols
• Reactions of primary, secondary and tertiary alcohols
• Animated mechanisms
• Reaction classification
• Reagents and conditions
• Structural equations
• Key definitions
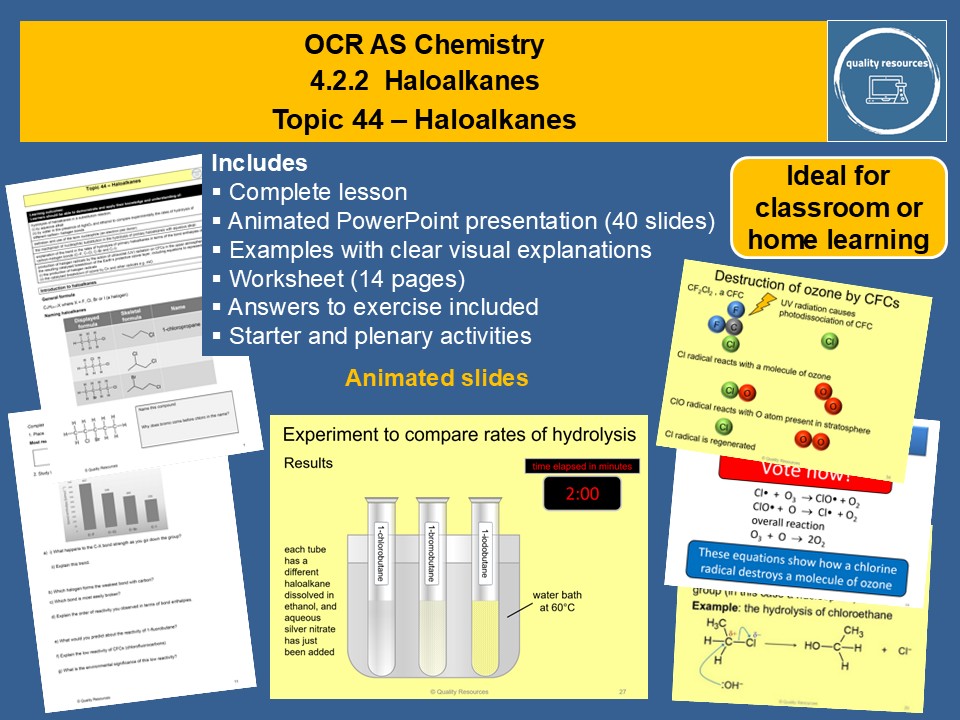
Haloalkanes
• Naming haloalkanes
• Reactivity of haloalkanes general equation for polymer formation
• Uses of haloalkanes
• Nucleophilic substitution reactions of haloalkanes including hydrolysis
• Mechanism of nucleophilic substitution
• Experiment to compare rates of hydrolysis of different haloalkanes
• Explaining the different rates of hydrolysis
• Organohalogen compounds and the environment
• Destruction of ozone by CFCs
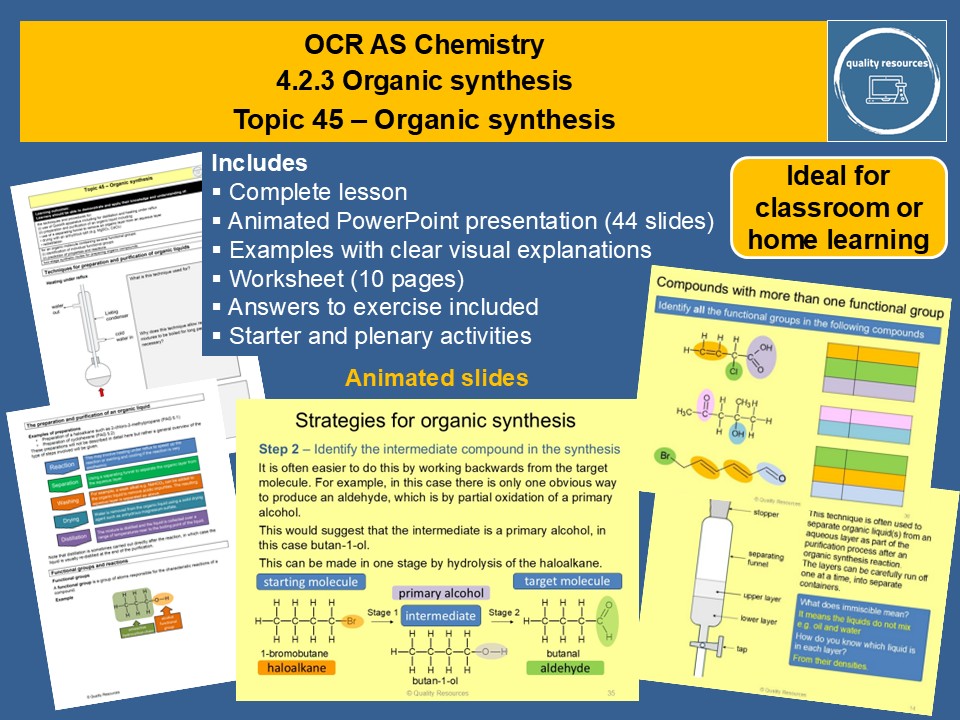
Organic synthesis
• Revision of functional groups
• Techniques for preparation and purification of organic liquids
• Heating under reflux
• Distillation
• Separation of immiscible liquids using a separating funnel
• Use of drying agents
• Stages in the preparation and purification of an organic liquid
• Tests for functional groups
• Compounds with more than one functional group
• Strategies for organic synthesis with examples
AS synthetic routes
• AS synthetic routes
• Animated mechanisms
• Key definitions
• Heating under reflux
• Distillation
• Reaction classification
• Reagents and conditions
• Structural equations
Infrared spectroscopy
• introduction to spectroscopy linked to the electromagnetic spectrum
• meaning of wavenumber and transmittance
• molecular vibrations
• bond stretching
• fingerprint region of spectrum
• types and shapes of peaks
• infrared and global warming
• the greenhouse effect and greenhouse gases
• interpreting the infrared spectrum
• examples of IR spectra with animated explanation linking peaks to structure
• uses of infrared spectroscopy
Mass spectrometry
• animated diagram and description of a mass spectrometer
• meaning of m/z
• relative intensity
• base peak
• molecular ion
• M+1 peak
• fragments
• interpreting the mass spectrum
• mass spectrum of ethanol
• animations of formation of fragments from ethanol
• summary of fragments for ethanol
• examples of mass spectra with animated explanation linking peaks to structure
• interpretation of mass spectrum of unknown compound leading to its identification
Identifying compounds from infrared and mass spectra
• Calculating empirical formula from % composition
• Calculating molecular formula from empirical formula and molar mass, using the molecular ion peak on the mass spectrum
• Using the infrared (IR) spectrum to identify bond stretches and hence functional group(s) present
• Drawing structural formulae consistent with the molecular formula and IR data
• Using mass spectrum to distinguish between the suggested structural formulae
• Identifying fragments in the mass spectrum
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.