Spec 1.1.5 - 6 The size and mass of atoms – One to two hours of teaching
Lesson(s) preparation:
Could print the questions on slides 19 & 21
Could prepare some isotopic mass calculation questions (I recommend chemsheets)
Could demonstrate these calculations using different mass apples of the same type (so exempla different massed atoms of the same element)
Rationale:
There is a recap of subatomic particles before moving onto isotopes and calculating relative atomic mass. The calculations are quite straight forward and two methods are shown in the slides, you may want to use both or just one. The amount you get through in the first lesson will depend on the ability of your class, the second lesson should be mainly practice of calculations.
Suggested teaching:
Lesson 1 (slides 2-17)
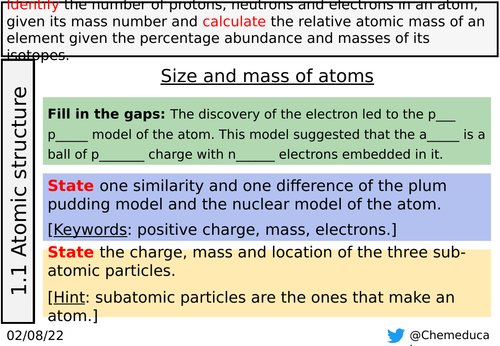
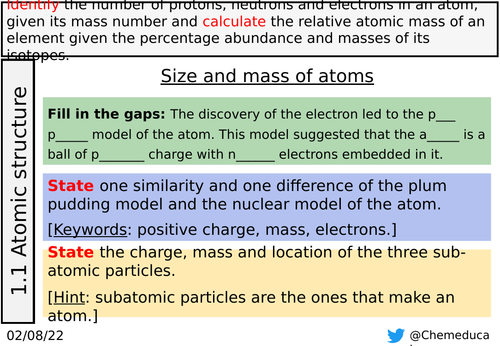
Slides 4-8: This is all appropriate recall from earlier in the topic. Slides 4 and 5 are the same but scaffolded with labels in slide 5.
Slides 9-14: Introducing the idea that atoms can have different masses, how to represent an calculate number of neutrons or mass number given appropriate information.
Slides 5-17: Standard method for calculating relative atomic mass, with a worked example and practice questions.
Lesson 2 (slides 18-26): There is an alternative method to calculating relative atomic mass, “without using an equation.” I have often found that a lot of students learn this method in maths.
Get this resource as part of a bundle and save up to 17%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.