5 Full Lesson Bundle which covers the lessons on aromatic compounds from the OCR A Level Chemistry Specification. See below for the lesson objectives
Lesson 1: Benzene and its Structure
- To describe the Kekulé model of benzene
- To describe the delocalised model of benzene in terms of P orbital overlap forming a delocalised π system
- To compare the Kekulé model of benzene and the delocalised model of benzene
- To explain the experimental evidence which supports the delocalised model of benzene in terms of bond lengths, enthalpy change of hydrogenation and resistance to reaction
Lesson 2: Naming Aromatic Compounds
- State the IUPAC name of substituted aromatic compounds
- Construct the structure of aromatic compounds based on their IUPAC names
- Analyse the correct numbering system for di and trisubstituted aromatic compounds
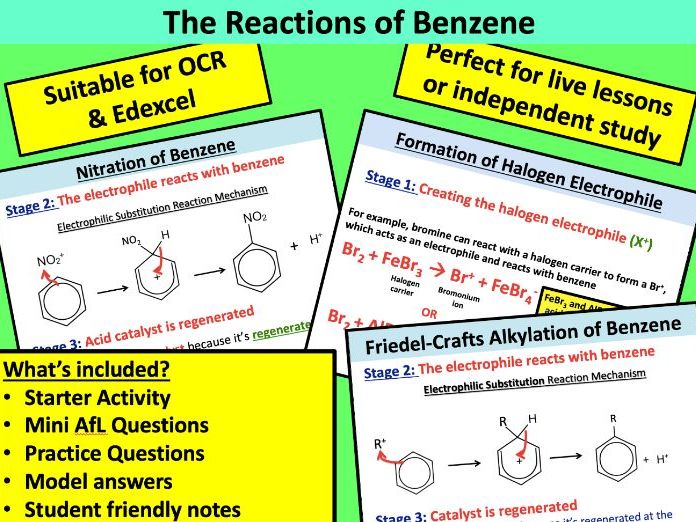
Lesson 3: The Reactions of Benzene
- To understand the electrophilic substitution of aromatic compounds with:
(i) concentrated nitric acid in the presence of concentrated sulfuric acid
(ii) a halogen in the presence of a halogen carrier
(iii) a haloalkane or acyl chloride in the presence of a halogen carrier (Friedel–Crafts reaction) and its importance to synthesis by formation of a C–C bond to an aromatic ring - To construct the mechanism of electrophilic substitution in arenes
Lesson 4: Phenols
- To recall and explain the electrophilic substitution reactions of phenol:
with bromine to form 2,4,6-tribromophenol
(ii) with dilute nitric acid to form a mixture of 2-nitrophenol and 4-nitrophenol
(j) To explain the relative ease of electrophilic substitution of phenol compared with benzene, in terms of electron pair donation to the π-system from an oxygen p-orbital in phenol - To understand the weak acidity of phenols shown by its neutralisation reaction with NaOH but absence of reaction with carbonates
Lesson 5: Directing Groups in Aromatic Compounds
-
To understand the 2- and 4-directing effect of electron- donating groups (OH, NH2) and the 3-directing effect of electron-withdrawing groups (NO2) in electrophilic substitution of aromatic compounds
-
To predict the substitution products of aromatic compounds by directing effects in organic synthesis
Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons including using your own lesson PowerPoints is a fundamental skill of a qualified/unqualified teacher that will be reviewed during these scenarios outlined above
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.