Equilibrium (OCR A Level Chemistry)
6 Full Lesson Bundle (includes a bonus lesson) on the topic of Equilibrium from the OCR A Level Chemistry specification plus an end of topic test. See below for the lessons and learning objectives
Lesson 1: Le Chatelier's Principle
1. To explain the term dynamic equilibrium
2. To apply le Chatelier’s principle to homogeneous equilibria in order to deduce qualitatively the effect of a change in temperature, pressure or concentration on the position of equilibrium
3. To explain why catalysts do not change the position of equilibrium
4. To explain the importance to the chemical industry of a compromise between chemical equilibrium and reaction rate in deciding the operational conditions
Lesson 2: The Equilibrium Constant Kc (Part 1)
1. To construct expressions for the equilibrium constant Kc for homogeneous reactions
2. To calculate the equilibrium constant Kc from provided equilibrium concentrations
3. To estimate the position of equilibrium from the magnitude of Kc
4. To know the techniques and procedures used to investigate changes to the position of equilibrium for changes in concentration and temperature
Lesson 3: The Equilibrium Constant Kc (Part 2)
1. To construct expressions for the equilibrium constant Kc for homogeneous and heterogeneous reactions
2. To calculate units for Kc
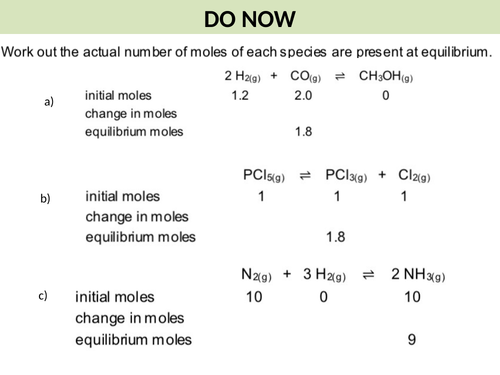
3. To calculate quantities present at equilibrium and therefore kc given appropriate data
Lesson 4: Controlling The Position of Equilibrium (Kc)
1. To understand and explain the effect of temperature, concentration, pressure and catalysts on Kc and controlling the position of equilibrium
Lesson 5: The Equilibrium Constant Kp
1. To use the terms mole fraction and partial pressure
2. To construct expressions for Kp for homogeneous and heterogeneous equilibria
3. To calculate Kp including determination of units
4. To understand the affect of temperature, pressure, concentration and catalysts on Kp and controlling the position of equilibrium
Lesson 6 (BONUS): Chemical Equilibirum (Practical Skills):
1. To understand how a titration experiment can be used to calculate the equilibrium constant, Kc
2. To understand how a colorimeter can be used to calculate the equilibrium constant, Kc
3. To analyse exam questions based on titration experiments in order to calculate out Kc
End of Topic Test:
A 45 minute end of chapter test on chemical equilibrium. The test covers content from both year 12 and 13 OCR on chemical equilibrium. A markscheme with model answers is also included which enables students self assess their answers in class with their teacher or as a homework task.
The test is based on the following learning objectives:
1. Apply le Chatelier’s principle to deduce qualitatively (from appropriate information) the effect of a change in temperature, concentration or pressure, on a homogeneous system in equilibrium.
2. Explain that a catalyst increases the rate of both forward and reverse reactions in an equilibrium by the same amount resulting in an unchanged position of equilibrium
3. Deduce, for homogeneous and heterogeneous reactions, expressions for the equilibrium constant Kc.
4. Calculate the values of the equilibrium constant, Kc (from provided or calculated equilibrium moles or concentrations), including determination of units.
5. Estimate the position of equilibrium from the magnitude of Kc.
6. Calculate, given appropriate data, the concentration or quantities present at equilibrium.
7. Deduce, for homogeneous and heterogeneous reactions, expressions for the equilibrium constant Kp.
8. Calculate the values of the equilibrium constant, Kp (from provided or calculated equilibrium moles or pressures), including determination of units.
9. Explain the effect of changing temperature on the value of Kc or Kp for exothermic and endothermic reactions.
10. State that the value of Kc or Kp is unaffected by changes in concentration or pressure or by the presence of a catalyst.
11. Explain how Kc or Kp controls the position of equilibrium on changing concentration, pressure and temperature
***Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons including using your own lesson PowerPoints is a fundamental skill of a qualified/unqualified teacher that will be reviewed during these scenarios outlined above***