
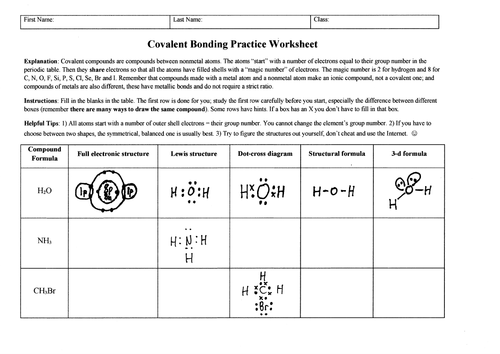
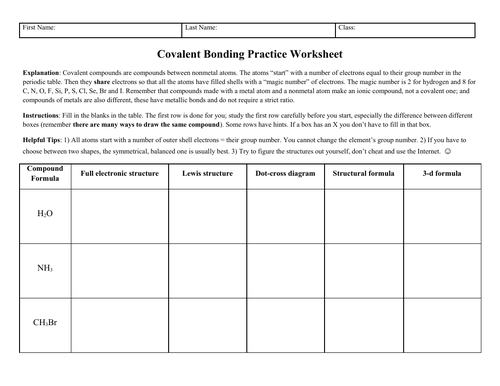
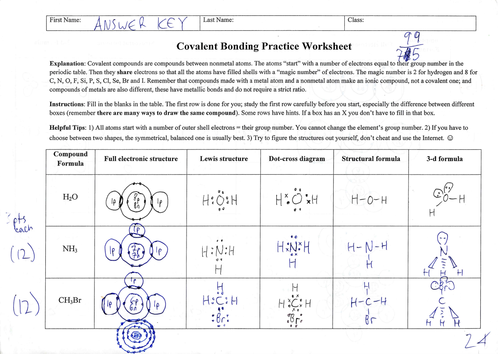
Covalently bound chemicals (i.e. molecules) can be depicted in many different ways, which is sometimes confusing for students: full electronic structures showing inner shells; Lewis structures; dot-cross structures; structural formulae as used in organic chemistry; and 3-d formulae which would be suitable for topics like VSEPR theory.
This worksheet is designed to provide carefully scaffolded practice with ALL of these ways simultaneously; it is intended not only to familiarize students with the different methods, but also to show them how they interrelate. Indirectly, the worksheet also shows why the methods are appropriate in different situations; for example, full electronic structures are cumbersome for large molecules containing heavy atoms, and 2-d structural formulae are not good for showing 3-d features such as bond angles.
Two versions of the worksheet are provided. The “complete” version has answers already in place for the first molecule, water. This is recommended for most classes since the completed example row shows what is required for the other rows. There is also a blank version supplied, since some teachers may prefer to work through the “water” row in class, with the students transcribing the structures as they appear. Lastly, a full mark scheme with point values is provided.
Level: mainly intended for IB Chemistry, the worksheet is also suitable for A-Level or AP Chemistry students, although in the latter case teachers may need to explain full electronic structures and dot-cross structures first, since these methods do not seem to be common in US chemistry curricula. In addition, I have successfully used it with KS3 groups (grades 9-10), including IGCSE and MYP sets, although these students will naturally require a little more support and pre-teaching of the theory.
Marking recommendations: 3 points for each correct structure with partial marks awarded for slightly defective answers. There are 99 marks available but I usually only require 75 with the extra available as “bonus points”, of course if your school’s assessment system does not allow this then it can be marked out of 99.
Differentiation: for lower attaining groups difficult problems like the full electronic structure for bromomethane, can be X’d out before duplicating the worksheet.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.