Electrolysis lesson looking at introducing students to the set up of electrolysis and the underlying ideas involved, including a quick recap of ionic compounds, metallic, non-metallic charges and word/symbol equations. From here, students develop an understanding of the origin of the word "electrolysis" and label the parts involved before describing the movement of ions and finally writing half equations:
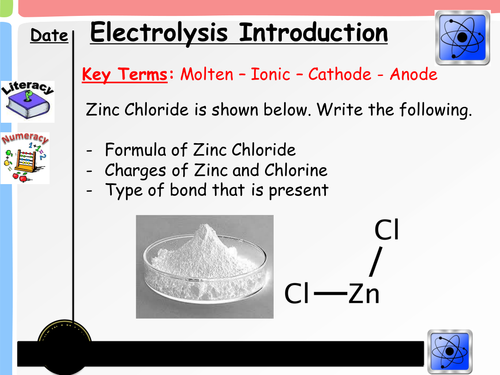
Starter - Students are reminded of the formula of ionic substances through the use of the starter questions. From here, it is important that they understand the charges of metal and non metal ions as a basic principle to apply later in the lesson
Intro/Recap -Building on from the starter, students familiarise themselves with word and symbol equations from previous topics.
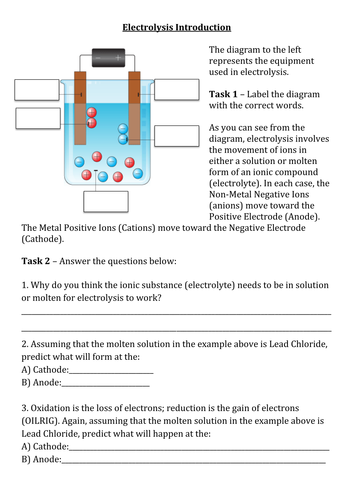
Main 1 - Youtube link in the notes section used to introduce electrolysis whilst introducing the origin of the word from ancient greek. Students then attempt tasks 1 + 2 on the worksheet before self-assessing their work.
Main 2 -Introduces half equations to the students in the form of a worked example. The example can then be used to help students complete task 3, which is writing half equations for the electrolysis of 3 basic ionic compounds and 1 challenge compound.
Plenary - Quick true of false activity summarising some learning points from the lesson.
Objectives:4 – WRITE a word equation to describe electrolysis
5 – DESCRIBE electrolysis in terms of movement of ions
6 – PREDICT the products at each electrode from electrolysis of a molten ionic compound (and complete a balanced half symbol equation)
Additional information is written in the notes section of each slide.
As always, and feedback is appreciated :)
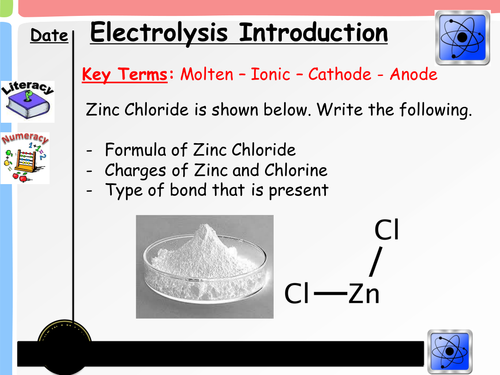
Starter - Students are reminded of the formula of ionic substances through the use of the starter questions. From here, it is important that they understand the charges of metal and non metal ions as a basic principle to apply later in the lesson
Intro/Recap -Building on from the starter, students familiarise themselves with word and symbol equations from previous topics.
Main 1 - Youtube link in the notes section used to introduce electrolysis whilst introducing the origin of the word from ancient greek. Students then attempt tasks 1 + 2 on the worksheet before self-assessing their work.
Main 2 -Introduces half equations to the students in the form of a worked example. The example can then be used to help students complete task 3, which is writing half equations for the electrolysis of 3 basic ionic compounds and 1 challenge compound.
Plenary - Quick true of false activity summarising some learning points from the lesson.
Objectives:4 – WRITE a word equation to describe electrolysis
5 – DESCRIBE electrolysis in terms of movement of ions
6 – PREDICT the products at each electrode from electrolysis of a molten ionic compound (and complete a balanced half symbol equation)
Additional information is written in the notes section of each slide.
As always, and feedback is appreciated :)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00