Entropy
Subject: Chemistry
Age range: 16+
Resource type: Assessment and revision





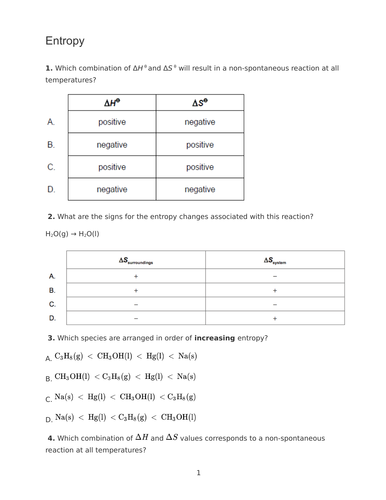
This 50 slide PowerPoint was planned as part of the IB schemes of work on Energy. They would also be suitable for other post-16 courses.
Included are fully completed PowerPoints including many examples, student versions of the PowerPoints with sections to complete independently and some exam style questions.
Topics covered include:
- Spontaneity and Disorder
- Entropy
- How to predict the sign of an entropy change
- Entropy across period 2
- Standard Entropy Change: ΔSθ
- Predicting whether a reaction will be spontaneous
- Calculating ΔSθ Universe
- Gibbs Free Energy
- At what temperature does a reaction become feasible?
- Gibbs Free Energy and Equilibrium
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.