New GCSE Chemistry lesson on Covalent bonding
Lesson starts with simple starter looking at different ways things are bonded together which allows discussion of hoe bonds vary in strength. Following a quick review goes over key content on ionic bonding.
The need for atoms to become chemically stable by gaining a full outer shell of electrons is introduced with illustrations of this. Different types of bonding are contrasted with picture representations to allow for comparisons to be made.
Covalent bonding is introduced by breaking down an explaining the words with key definitions behind these.
Atoms numbers are reviewed and linked to electrons in outer shell. Chlorine is provided as an example of dot and cross diagram for covalent boding.
Properties of covalent bonds are also reviewed.
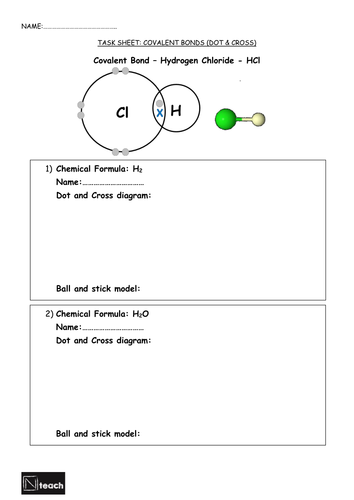
Students are prompted to draw dot and cross diagrams for a variety of key substances.
Lesson Objectives:
1) Describe how covalent bonds are formed.
2) Recognise covalent compounds from their formula, name or bonding diagram.
3) Draw dot and cross diagrams/ball and stick diagrams for familiar molecules.
4) Suggest how properties vary for double covalent bonds and single covalent bonds.
Additions to lesson made with worksheet as promised: 06/09/16
Further updates to follow
More chemistry lessons to be listed on Nteach soon.
Lesson starts with simple starter looking at different ways things are bonded together which allows discussion of hoe bonds vary in strength. Following a quick review goes over key content on ionic bonding.
The need for atoms to become chemically stable by gaining a full outer shell of electrons is introduced with illustrations of this. Different types of bonding are contrasted with picture representations to allow for comparisons to be made.
Covalent bonding is introduced by breaking down an explaining the words with key definitions behind these.
Atoms numbers are reviewed and linked to electrons in outer shell. Chlorine is provided as an example of dot and cross diagram for covalent boding.
Properties of covalent bonds are also reviewed.
Students are prompted to draw dot and cross diagrams for a variety of key substances.
Lesson Objectives:
1) Describe how covalent bonds are formed.
2) Recognise covalent compounds from their formula, name or bonding diagram.
3) Draw dot and cross diagrams/ball and stick diagrams for familiar molecules.
4) Suggest how properties vary for double covalent bonds and single covalent bonds.
Additions to lesson made with worksheet as promised: 06/09/16
Further updates to follow
More chemistry lessons to be listed on Nteach soon.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00