



Note re-numbering - if you have already downloaded this resource you can download again to get the updated version.
My resources now cover the whole of OCR AS Chemistry.
Each download includes a list of all available lessons and bundles.
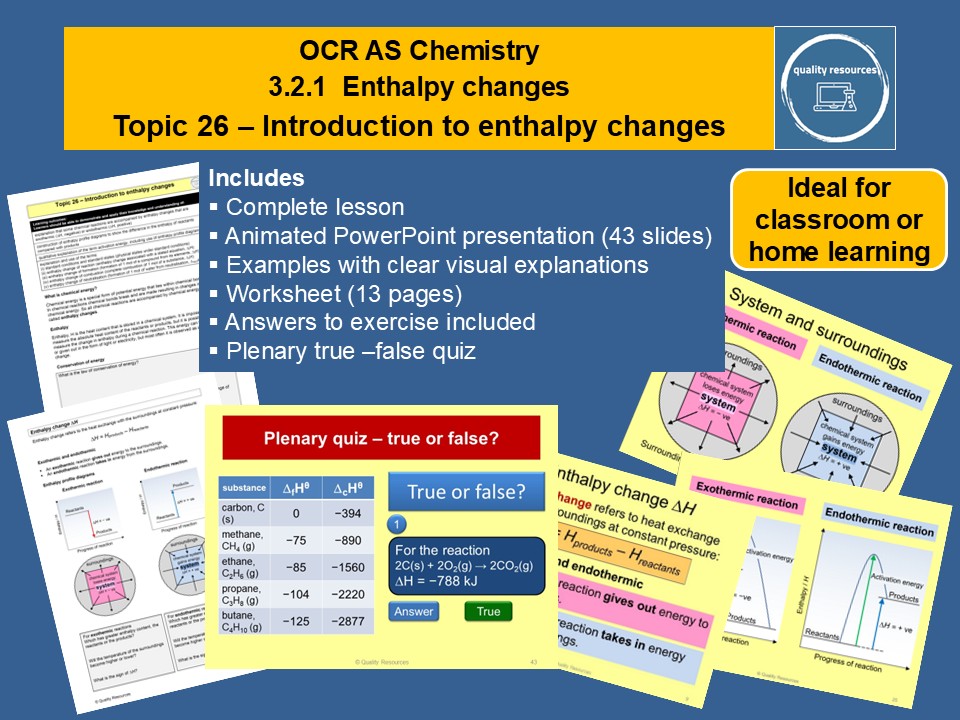
This complete year 12 lesson on enthalpy changes covers part of OCR section 3.2.1 (Enthalpy changes). It includes the energetics topics: exothermic and endothermic reactions, enthalpy profile diagrams and definitions of enthalpy changes with their equations. It features a 43 slide interactive PowerPoint that illustrates the concepts in a lively, visual and systematic way and includes a starter, learning checks, clearly explained examples and a plenary quiz. A 13 page worksheet and answers to the exercise are provided.
This resource is part of a series covering the OCR AS Chemistry specification and relates to the following sections:
Module 3 – Periodic table and energy
Part 2 – Physical chemistry
3.2.1 – Enthalpy changes
If you find this free resource useful please leave a review!
Content covered:
• What is enthalpy
• Law of conservation of energy
• Enthalpy change
• Enthalpy profile diagrams
• System and surroundings
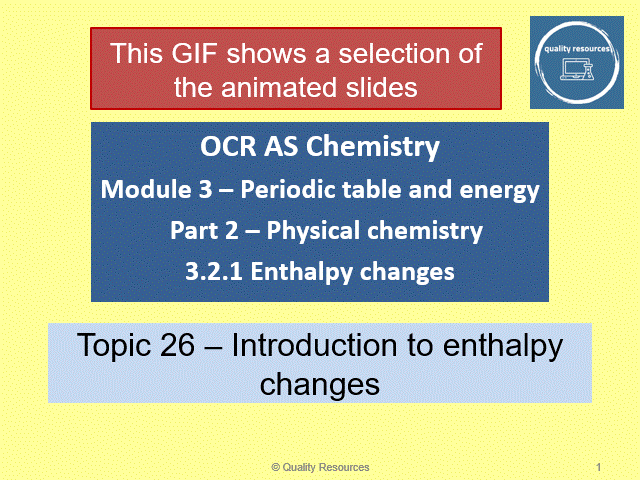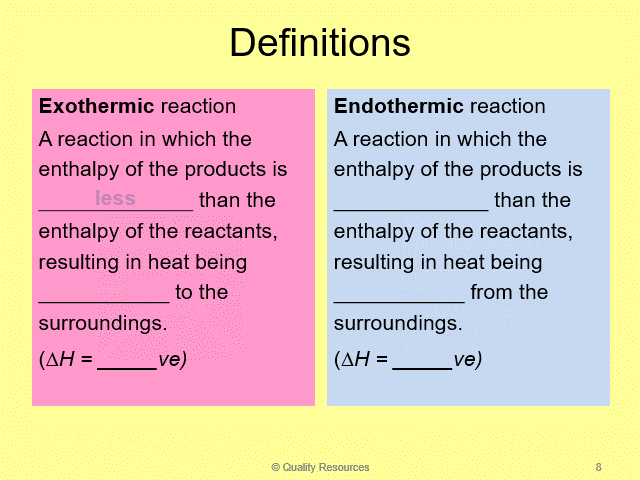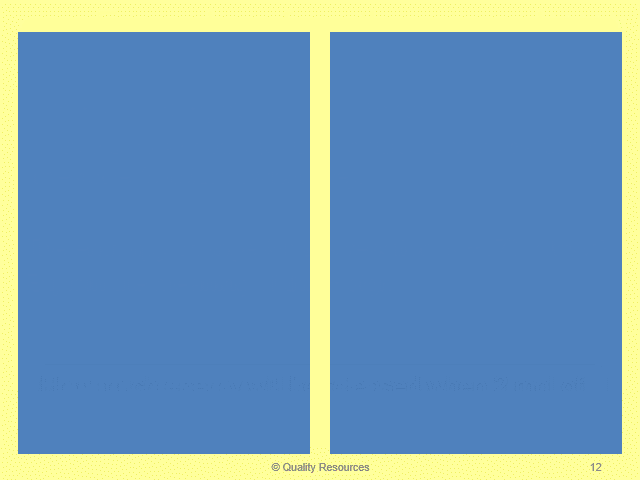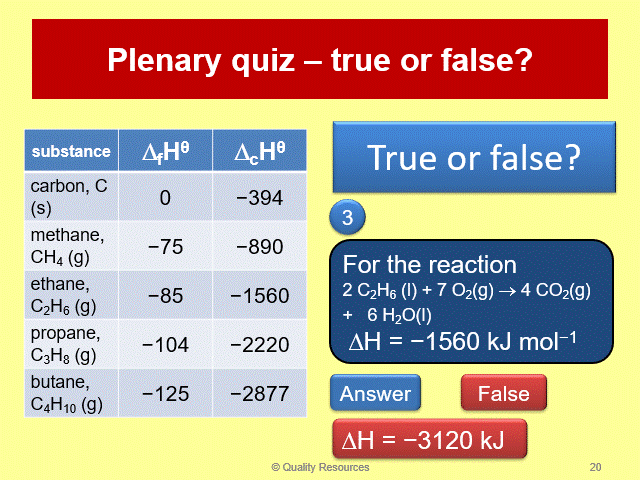
• Exothermic and endothermic reactions and examples
• Activation energy
• Standard enthalpy changes
• Standard conditions
• Definitions of enthalpy changes and their equations
• Simple calculations involving enthalpy changes
Duration: 1 lesson
Links
Previous topic: Topic 25 – Qualitative analysis
https://www.tes.com/teaching-resource/qualitative-analysis-ocr-as-chemistry-13128777
Next topic: Topic 27 – Experimental determination of enthalpy changes
https://www.tes.com/teaching-resource/experimental-determination-of-enthalpy-changes-ocr-as-chemistry-12517135
Free resource - standard form, decimal places and significant figures
https://www.tes.com/teaching-resource/resource-12405507
Get this resource as part of a bundle and save up to 42%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Periodic table and energy
*Note re-numbering - if you have already paid for this resource you can download it again to get the updated version without further payment.* **My resources now cover the whole of OCR AS Chemistry.** Each download includes a list of all available lessons and bundles. This bundle covers all of the OCR A level chemistry specification module 3. – periodic table and energy. Resources included are: • Periodic table past and present • Periodicity of ionisation energies and melting points • Group 2 • The halogens • Qualitative analysis • Introduction to enthalpy changes • Experimental determination of enthalpy changes • Bond enthalpies • Hess’ Law • Measuring rate of reaction • Collision theory and factors affecting rate of reaction • Introduction to chemical equilibrium • Le Chatelier’s principle and equilibrium constant Kc Each topic includes a fully interactive PowerPoint including starter, group activities, questions and plenary along with a worksheet. Answers to all exercises are provided. Some of the resources include a PowerPoint quiz and all are ideal for classroom or home learning. This bundle is part of a series covering the OCR AS Chemistry specification and relates to the following section: Module 3 – Periodic table and energy (all) Part 1 – The periodic table 3.1.1 Periodicity 3.1.2 Group 2 3.1.3 The halogens 3.1.4 Qualitative analysis Part 2 Physical chemistry 3.2.1 Enthalpy changes 3.2.2 Reaction rates 3.2.3 Chemical equilibrium **Please review!** **Content covered:** **Periodic table past and present** • The history of the periodic table, including Newlands’ and Mendeleev’s contributions • The structure of the modern periodic table – periods, groups and blocks • Relationship between electron configuration and the periodic table • Periodicity – the variation in properties when plotted against atomic number • Periodicity and metallic character • Metal and non-metals **Periodicity of ionisation energies and melting point** • First ionisation energy – definition • Factors affecting ionisation energies: nuclear charge, atomic radius and shielding • Explanation of shielding • Successive ionisation energies • Predicting group from successive ionisation energies • Periodicity of first ionisation energies • Trends across a period and down a group • Explanations of small decreases from group 2 to group 3 and from group 5 to group 6 • Periodicity of structure of elements: giant metallic, giant covalent and simple molecular structures • Periodicity of melting points and explanation in terms of structure. **Group 2** • Structure and physical properties of group 2 elements • Electron configuration and formation of ions • First ionisation enthalpy and reactivity • Redox reactions of group 2 metals with oxygen, water and acids • Properties of group 2 compounds • Group 2 oxides and hydroxides – reactions with acids • Group 2 oxides – reaction with water • Solubility and alkalinity of group 2 hydroxides • Reactions of group 2 carbonates with acid • Uses of group 2 compounds **The halogens** • Structure and physical properties of group 17 elements • Electron configuration and formation of ions • Redox reactions of halogens • Reactivity of halogens • Displacement reactions • Disproportionation reactions of the halogens, including production of bleach • Benefits and hazards of treating drinking water with chlorine • Precipitation reactions of aqueous halide ions with aqueous silver nitrate • Use of silver nitrate as a test for aqueous halide ions **Qualitative analysis** • Precipitation and acid-base reactions • Tests for carbon dioxide and ammonia • Tests for anions including carbonate, sulfate, chloride, bromide and iodide ions • Sequence of tests for anions, with reasons • Analysing mixtures of anions • Tests for cations – ammonium ion **Enthalpy changes** • What is enthalpy • Law of conservation of energy • Enthalpy change • Enthalpy profile diagrams • System and surroundings • Exothermic and endothermic reactions and examples • Activation energy • Standard enthalpy changes • Standard conditions • Definitions of enthalpy changes • Simple calculations involving enthalpy changes **Experimental determination of enthalpy changes** • Energy exchange with the surroundings - heat loss in a chemical system = heat gain by surroundings • Temperature scales • Determining enthalpy changes using calorimetry • Calculations involving q = mc∆T . • Determining enthalpy changes in solution • Determining enthalpy of combustion • Errors associated with calorimetry experiments and how to minimise them • Cooling curves and how to find the temperature rise • Thermometric titration **Bond enthalpies** • Making covalent bonds (exothermic) and breaking covalent bonds (endothermic) • Overall enthalpy change linked to relative enthalpies of breaking and making bonds – enthalpy profile diagram • Average bond enthalpies and why they differ from actual bond enthalpies • Factors affecting average bond enthalpies • Calculations involving bond enthalpies • Limitations of bond enthalpy calculations • Plenary discussion about why there is a constant increase in the enthalpy change of combustion of alcohols for each CH2 group added **Hess’ Law** • Hess’ Law • Indirect determination of enthalpy changes • Enthalpy cycles • Calculating enthalpy changes from enthalpy changes of combustion • Calculating enthalpy changes from enthalpy changes of formation • Summary of types of enthalpy calculation • Calculating enthalpy changes from unfamiliar enthalpy cycles **Measuring rate of reaction** • Gradients • Definition , calculation and units of rate of reaction • Measuring rate of reaction experimentally using volume of gas, loss of mass or change in concentration • Determining rate from a graph of concentration (or gas volume or mass loss) against time using tangents • Smooth curve versus dot-to-dot **Collision theory and factors affecting rate of reaction** • Factors affecting rate of reaction • The collision theory of reactions • Activation energy and enthalpy profile diagrams • Effect of concentration and pressure on rate and explanation in terms of collision theory • Effect of temperature and catalysts on rate • Catalysts – how they work and their advantages • Using Boltzmann distribution curves and activation energy to explain the effect of temperature and catalysts on rate **Introduction to chemical equilibrium** • Static vs dynamic equilibrium • Irreversible and reversible reactions • Meaning of closed system • Examples of dynamic equilibrium and how it is reached • Definition of dynamic equilibrium • How rates vary with time (graph of rate against time) • How concentrations vary with time (graphs of concentration against time) • Position of equilibrium – illustrated by concentration-time graphs • Yield of reaction **Le Chatelier’s principle and equilibrium constant Kc** • Le Chatelier’s principle • Effect of changing concentration, pressure or temperature on position of equilibrium, predicted and explained using le Chatelier’s principle • Practical examples with colour changes • Effect of adding a catalyst on rate of reaction and position of equilibrium • Position of equilibrium and yield • Choice of conditions in the chemical industry - factors considered including yield, rate , costs and safety • How far, how fast? • The Haber process as example of an industrial process • The equilibrium constant Kc • The equilibrium law • Writing expressions for Kc and calculating values. **Links** Next topic: – Topic 34 - Introduction to organic chemistry (free resource) https://www.tes.com/teaching-resource/introduction-to-organic-chemistry-ocr-as-chemistry-12237143 Next large bundle: Core organic chemistry https://www.tes.com/teaching-resource/core-organic-chemistry-bundle-13353996
Physical chemistry - enthalpy, rate and equilibrium bundle
*Note re-numbering - if you have already paid for this resource you can download it again to get the updated version without further payment.* **My resources now cover the whole of OCR AS Chemistry.** Each download includes a list of all available lessons and bundles. This bundle covers all of the OCR A level chemistry specification section 3.2. – physical chemistry. Resources include are • Introduction to enthalpy changes • Experimental determination of enthalpy changes • Bond enthalpies • Hess’ Law • Measuring rate of reaction • Collision theory and factors affecting rate of reaction • Introduction to chemical equilibrium • Le Chatelier’s principle and equilibrium constant Kc Each topic includes a fully interactive PowerPoint including starter, group activities, questions and plenary along with a worksheet. Answers to all exercises are provided. Some of the resources include a PowerPoint quiz and all are ideal for classroom or home learning. This bundle is part of a series covering the OCR AS Chemistry specification and relates to the following section: Module 3 – Periodic table and energy Part 2 – Physical chemistry 3.2.1 Enthalpy changes 3.2.2 Reaction rates 3.2.3 Chemical equilibrium **Please review!** **Content covered:** **Enthalpy changes** • What is enthalpy • Law of conservation of energy • Enthalpy change • Enthalpy profile diagrams • System and surroundings • Exothermic and endothermic reactions and examples • Activation energy • Standard enthalpy changes • Standard conditions • Definitions of enthalpy changes • Simple calculations involving enthalpy changes **Experimental determination of enthalpy changes** • Energy exchange with the surroundings - heat loss in a chemical system = heat gain by surroundings • Temperature scales • Determining enthalpy changes using calorimetry • Calculations involving q = mc∆T . • Determining enthalpy changes in solution • Determining enthalpy of combustion • Errors associated with calorimetry experiments and how to minimise them • Cooling curves and how to find the temperature rise • Thermometric titration **Bond enthalpies** • Making covalent bonds (exothermic) and breaking covalent bonds (endothermic) • Overall enthalpy change linked to relative enthalpies of breaking and making bonds – enthalpy profile diagram • Average bond enthalpies and why they differ from actual bond enthalpies • Factors affecting average bond enthalpies • Calculations involving bond enthalpies • Limitations of bond enthalpy calculations • Plenary discussion about why there is a constant increase in the enthalpy change of combustion of alcohols for each CH2 group added **Hess’ Law** • Hess’ Law • Indirect determination of enthalpy changes • Enthalpy cycles • Calculating enthalpy changes from enthalpy changes of combustion • Calculating enthalpy changes from enthalpy changes of formation • Summary of types of enthalpy calculation • Calculating enthalpy changes from unfamiliar enthalpy cycles **Measuring rate of reaction** • Gradients • Definition , calculation and units of rate of reaction • Measuring rate of reaction experimentally using volume of gas, loss of mass or change in concentration • Determining rate from a graph of concentration (or gas volume or mass loss) against time using tangents • Smooth curve versus dot-to-dot **Collision theory and factors affecting rate of reaction** • Factors affecting rate of reaction • The collision theory of reactions • Activation energy and enthalpy profile diagrams • Effect of concentration and pressure on rate and explanation in terms of collision theory • Effect of temperature and catalysts on rate • Catalysts – how they work and their advantages • Using Boltzmann distribution curves and activation energy to explain the effect of temperature and catalysts on rate **Introduction to chemical equilibrium** • Static vs dynamic equilibrium • Irreversible and reversible reactions • Meaning of closed system • Examples of dynamic equilibrium and how it is reached • Definition of dynamic equilibrium • How rates vary with time (graph of rate against time) • How concentrations vary with time (graphs of concentration against time) • Position of equilibrium – illustrated by concentration-time graphs • Yield of reaction **Le Chatelier’s principle and equilibrium constant Kc** • Le Chatelier’s principle • Effect of changing concentration, pressure or temperature on position of equilibrium, predicted and explained using le Chatelier’s principle • Practical examples with colour changes • Effect of adding a catalyst on rate of reaction and position of equilibrium • Position of equilibrium and yield • Choice of conditions in the chemical industry - factors considered including yield, rate , costs and safety • How far, how fast? • The Haber process as example of an industrial process • The equilibrium constant Kc • The equilibrium law • Writing expressions for Kc and calculating values. **Links** Next topic: – Topic 34 - Introduction to organic chemistry (free resource) https://www.tes.com/teaching-resource/introduction-to-organic-chemistry-ocr-as-chemistry-12237143 Next medium bundle: Basic concepts and hydrocarbons https://www.tes.com/teaching-resource/basic-concepts-and-hydrocarbons-bundle-ocr-as-chemistry-13316905
Enthalpy changes bundle OCR AS Chemistry
*Note re-numbering - if you have already paid for this resource you can download it again to get the updated version without further payment.* **My resources now cover the whole of OCR AS Chemistry.** Each download includes a list of all available lessons and bundles. This bundle covers all of the OCR A level chemistry specification section 3.1 (the periodic table). The resources included are • Periodic table past and present • Periodicity of ionisation energies and melting points • Group 2 • The halogens • Qualitative analysis Each topic includes a fully interactive PowerPoint including starter, group activities, questions and plenary along with a worksheet. Answers to all exercises are provided. Some of the resources include a PowerPoint quiz and all are ideal for classroom or home learning. This bundle is part of a series covering the OCR AS Chemistry specification and relates to the following section: Module 3 – Periodic table and energy / Part 2 – Physical chemistry / 3.2.1 Enthalpy changes **Please review!** **Content covered:** **Enthalpy changes** • What is enthalpy • Law of conservation of energy • Enthalpy change • Enthalpy profile diagrams • System and surroundings • Exothermic and endothermic reactions and examples • Activation energy • Standard enthalpy changes • Standard conditions • Definitions of enthalpy changes • Simple calculations involving enthalpy changes **Experimental determination of enthalpy changes** • Energy exchange with the surroundings - heat loss in a chemical system = heat gain by surroundings • Temperature scales • Determining enthalpy changes using calorimetry • Calculations involving q = mc∆T . • Determining enthalpy changes in solution • Determining enthalpy of combustion • Errors associated with calorimetry experiments and how to minimise them • Cooling curves and how to find the temperature rise • Thermometric titration **Bond enthalpies** • Making covalent bonds (exothermic) and breaking covalent bonds (endothermic) • Overall enthalpy change linked to relative enthalpies of breaking and making bonds – enthalpy profile diagram • Average bond enthalpies and why they differ from actual bond enthalpies • Factors affecting average bond enthalpies • Calculations involving bond enthalpies • Limitations of bond enthalpy calculations • Plenary discussion about why there is a constant increase in the enthalpy change of combustion of alcohols for each CH2 group added **Hess’ Law** • Hess’ Law • Indirect determination of enthalpy changes • Enthalpy cycles • Calculating enthalpy changes from enthalpy changes of combustion • Calculating enthalpy changes from enthalpy changes of formation • Summary of types of enthalpy calculation • Calculating enthalpy changes from unfamiliar enthalpy cycles **Links** Next topic: Topic 30 – Measuring rate of reaction https://www.tes.com/teaching-resource/measuring-rate-of-reaction-ocr-as-chemistry-13180177 Next small bundle: Reaction rates and chemical equilibrium https://www.tes.com/teaching-resource/reaction-rates-and-chemical-equilibrium-bundle-13214197
Something went wrong, please try again later.
Thank you. Excellent resource
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.