Note re-numbering - if you have already paid for this resource you can download it again to get the updated version without further payment.
My resources now cover the whole of OCR AS Chemistry.
Each download includes a list of all available lessons and bundles.
This bundle covers all of the OCR A level chemistry specification section 3.1 (the periodic table).
The resources included are
• Periodic table past and present
• Periodicity of ionisation energies and melting points
• Group 2
• The halogens
• Qualitative analysis
Each topic includes a fully interactive PowerPoint including starter, group activities, questions and plenary along with a worksheet. Answers to all exercises are provided. Some of the resources include a PowerPoint quiz and all are ideal for classroom or home learning.
This bundle is part of a series covering the OCR AS Chemistry specification and relates to the following section:
Module 3 – Periodic table and energy / Part 2 – Physical chemistry / 3.2.1 Enthalpy changes
Please review!
Content covered:
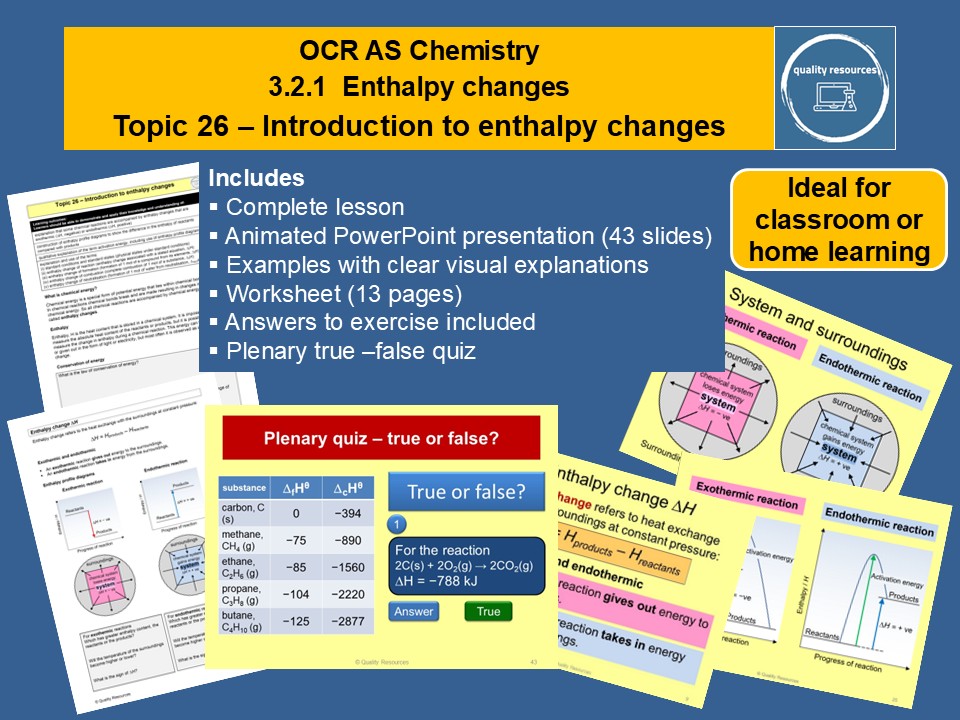
Enthalpy changes
• What is enthalpy
• Law of conservation of energy
• Enthalpy change
• Enthalpy profile diagrams
• System and surroundings
• Exothermic and endothermic reactions and examples
• Activation energy
• Standard enthalpy changes
• Standard conditions
• Definitions of enthalpy changes
• Simple calculations involving enthalpy changes
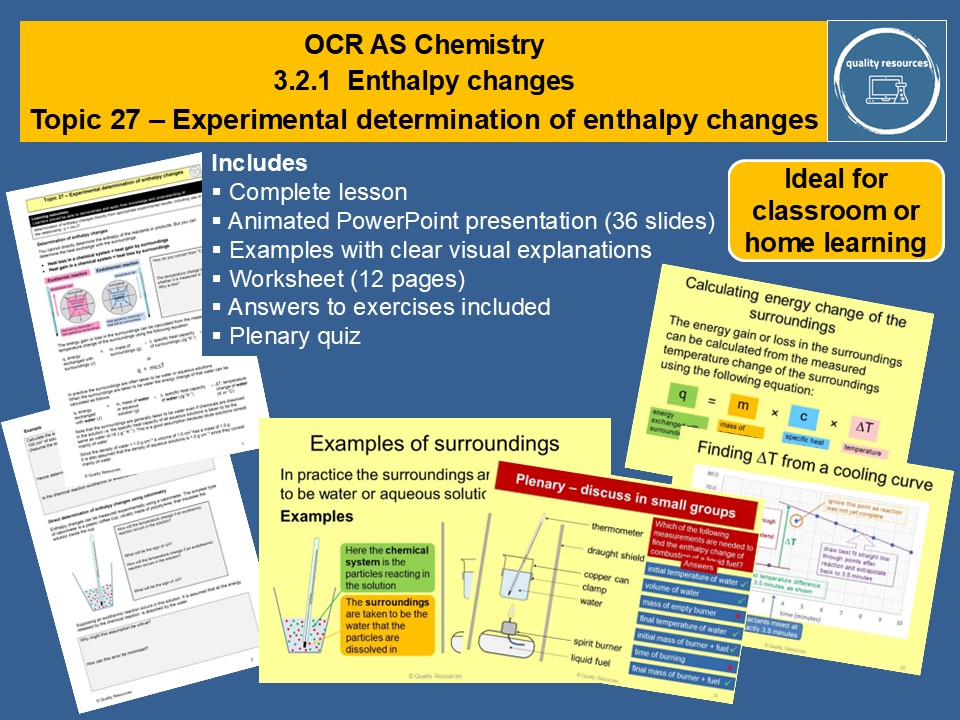
Experimental determination of enthalpy changes
• Energy exchange with the surroundings - heat loss in a chemical system = heat gain by surroundings
• Temperature scales
• Determining enthalpy changes using calorimetry
• Calculations involving q = mc∆T .
• Determining enthalpy changes in solution
• Determining enthalpy of combustion
• Errors associated with calorimetry experiments and how to minimise them
• Cooling curves and how to find the temperature rise
• Thermometric titration
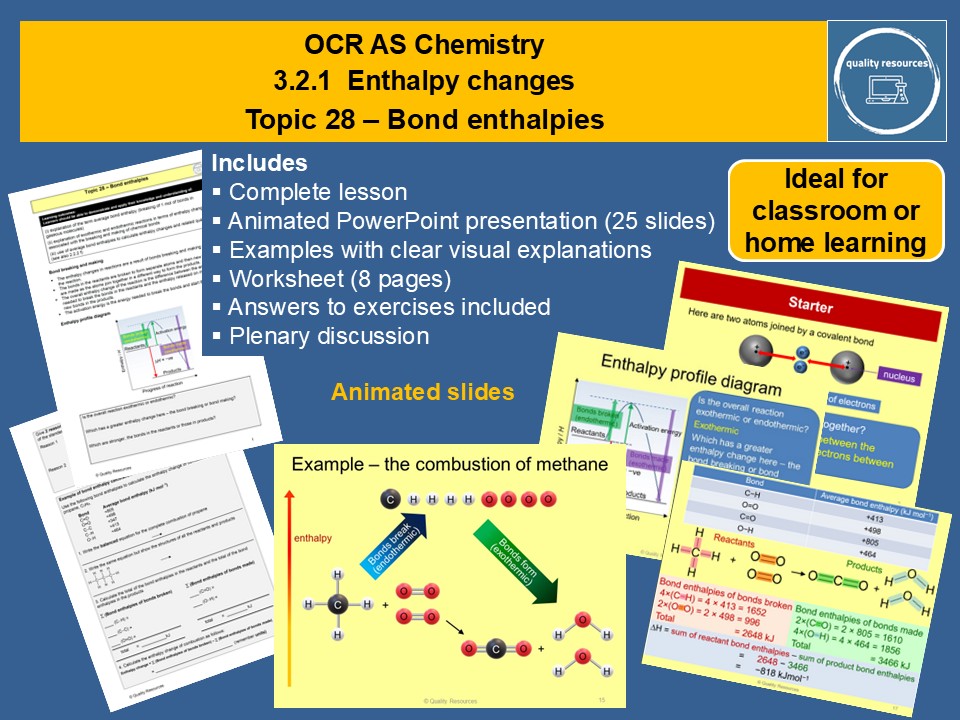
Bond enthalpies
• Making covalent bonds (exothermic) and breaking covalent bonds (endothermic)
• Overall enthalpy change linked to relative enthalpies of breaking and making bonds – enthalpy profile diagram
• Average bond enthalpies and why they differ from actual bond enthalpies
• Factors affecting average bond enthalpies
• Calculations involving bond enthalpies
• Limitations of bond enthalpy calculations
• Plenary discussion about why there is a constant increase in the enthalpy change of combustion of alcohols for each CH2 group added
Hess’ Law
• Hess’ Law
• Indirect determination of enthalpy changes
• Enthalpy cycles
• Calculating enthalpy changes from enthalpy changes of combustion
• Calculating enthalpy changes from enthalpy changes of formation
• Summary of types of enthalpy calculation
• Calculating enthalpy changes from unfamiliar enthalpy cycles
Links
Next topic:
Topic 30 – Measuring rate of reaction
https://www.tes.com/teaching-resource/measuring-rate-of-reaction-ocr-as-chemistry-13180177
Next small bundle:
Reaction rates and chemical equilibrium
https://www.tes.com/teaching-resource/reaction-rates-and-chemical-equilibrium-bundle-13214197
Something went wrong, please try again later.
I'm new to AS Chem and this has been so helpful! Would definitely be interested in AS Equilibria and Kinetics bundles too if they're ever available :)
sequenced well with clear content - great resource!
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.