Note re-numbering - if you have already paid for this resource you can download it again to get the updated version without further payment.
My resources now cover the whole of OCR AS Chemistry.
Each download includes a list of all available lessons and bundles.
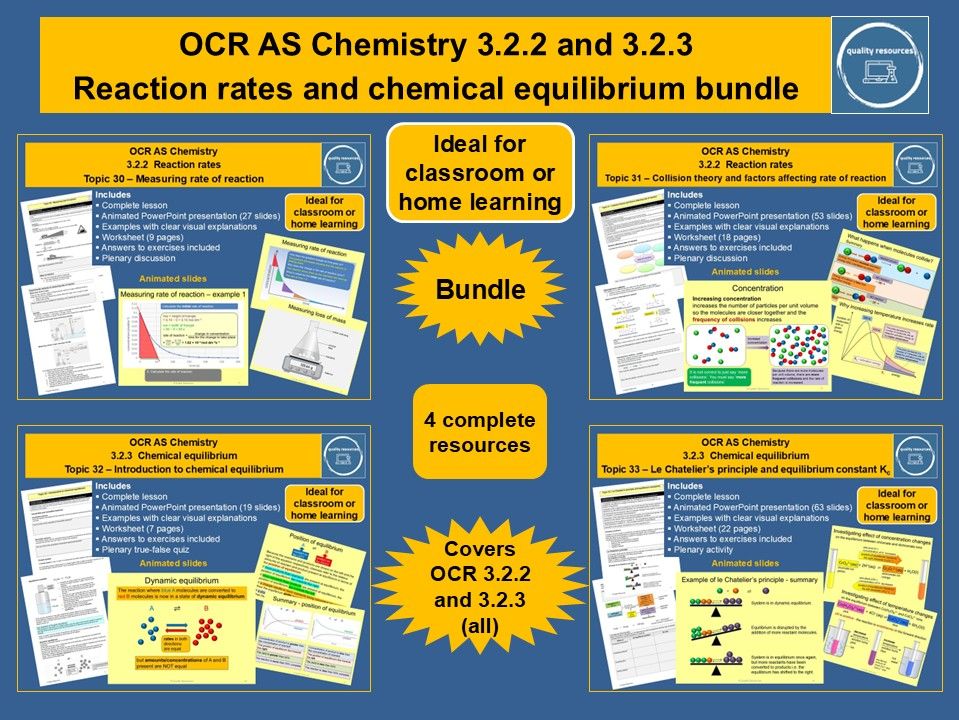
This bundle covers all of the OCR A level chemistry specification sections 3.2.2 (reaction rates) and 3.2.3 (chemical equilibrium).
Resources included are
• Measuring rate of reaction
• Collision theory and factors affecting rate of reaction
• Introduction to chemical equilibrium
• Le Chatelier’s principle and equilibrium constant Kc
Each topic includes a fully interactive PowerPoint including starter, activities, questions and plenary along with a worksheet. Answers to all exercises are provided. Some of the resources include a PowerPoint quiz and all are ideal for classroom or home learning.
This bundle is part of a series covering the OCR AS Chemistry specification and relates to the following section:
Module 3 – Periodic table and energy
Part 2 – Physical chemistry –
3.2.2 Reaction rates
3.2.3 Chemical equilibrium
Please review!
Content covered:
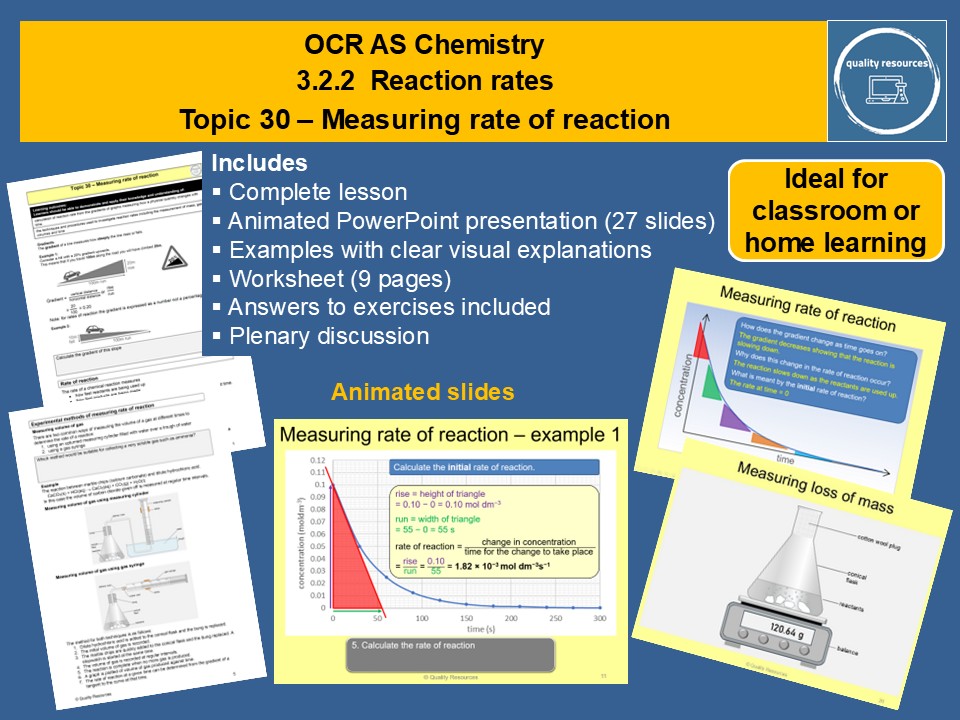
Measuring rate of reaction
• Gradients
• Definition , calculation and units of rate of reaction
• Measuring rate of reaction experimentally using volume of gas, loss of mass or change in concentration
• Determining rate from a graph of concentration (or gas volume or mass loss) against time using tangents
• Smooth curve versus dot-to-dot
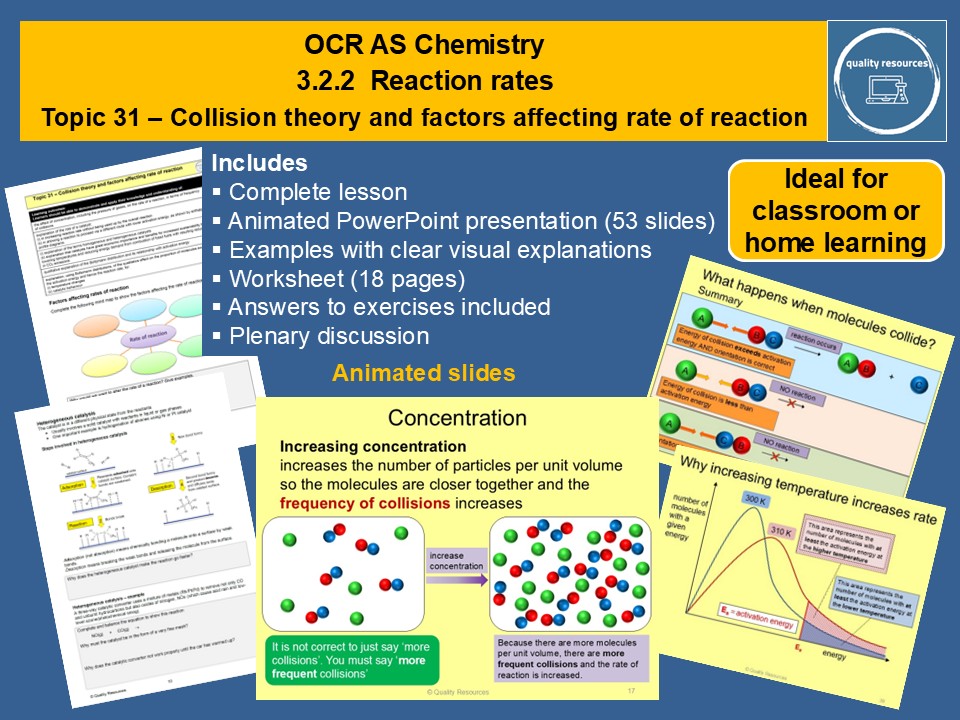
Collision theory and factors affecting rate of reaction
• Factors affecting rate of reaction
• The collision theory of reactions
• Activation energy and enthalpy profile diagrams
• Effect of concentration and pressure on rate and explanation in terms of collision theory
• Effect of temperature and catalysts on rate
• Catalysts – how they work and their advantages
• Using Boltzmann distribution curves and activation energy to explain the effect of temperature and catalysts on rate
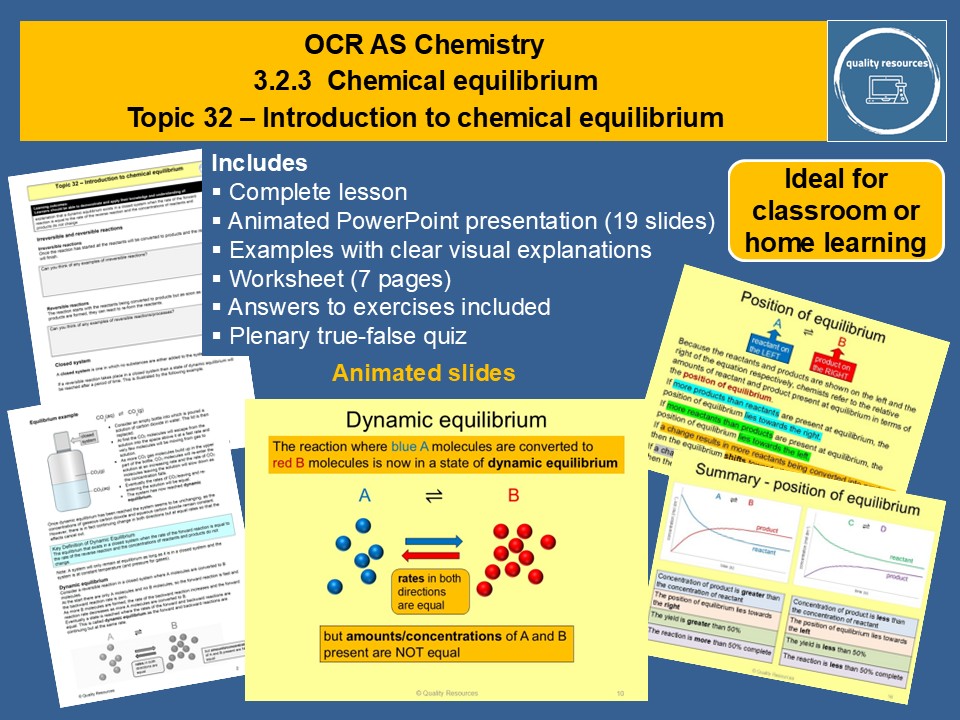
Introduction to chemical equilibrium
• Static vs dynamic equilibrium
• Irreversible and reversible reactions
• Meaning of closed system
• Examples of dynamic equilibrium and how it is reached
• Definition of dynamic equilibrium
• How rates vary with time (graph of rate against time)
• How concentrations vary with time (graphs of concentration against time)
• Position of equilibrium – illustrated by concentration-time graphs
• Yield of reaction
Le Chatelier’s principle and equilibrium constant Kc
• Le Chatelier’s principle
• Effect of changing concentration, pressure or temperature on position of equilibrium, predicted and explained using le Chatelier’s principle
• Practical examples with colour changes
• Effect of adding a catalyst on rate of reaction and position of equilibrium
• Position of equilibrium and yield
• Choice of conditions in the chemical industry - factors considered including yield, rate , costs and safety
• How far, how fast?
• The Haber process as example of an industrial process
• The equilibrium constant Kc
• The equilibrium law
• Writing expressions for Kc and calculating values.
Links
Next topic: – Topic 34 - Introduction to organic chemistry (free resource)
https://www.tes.com/teaching-resource/introduction-to-organic-chemistry-ocr-as-chemistry-12237143
Next small bundle: Basic concepts of organic chemistry
https://www.tes.com/teaching-resource/basic-concepts-of-organic-chemistry-ocr-as-chemistry-12643964
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.