
LESSON OBJECTIVE: Understand the concept of electron affinity and use Born-Haber cycles to calculate lattice energies
Learning Outcomes:
(taken from the Cambridge International AS and A Level Chemistry curriculum)
23.1 Lattice energy and Born-Haber cycles
-
define and use the terms:
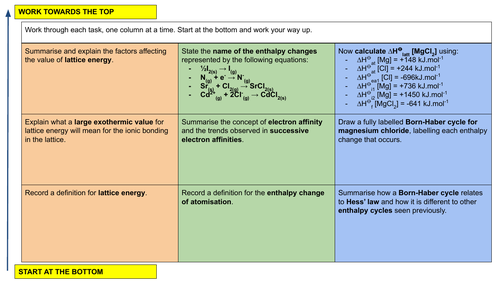
a) enthalpy change of atomisation, ΔHat
b) lattice energy, ΔHlatt (the change from gas phase ions to solid lattice) -
a) define and use the term first electron affinity, EA
b) explain the factors affecting the electron affinities of elements
c) describe and explain the trends in the electron affinities of the Group 16 and Group 17 elements -
construct and use Born–Haber cycles for ionic solids (limited to +1 and +2 cations, –1 and –2 anions)
-
carry out calculations involving Born–Haber cycles
-
explain, in qualitative terms, the effect of ionic charge and of ionic radius on the numerical magnitude of a lattice energy
Get this resource as part of a bundle and save up to 50%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.