

This PowerPoint was planned as part of the IB scheme of work on Organic Chemistry, and covers some of the necessary content for the Higher Level topics. It would also be suitable for other post-16 courses.
Included are the fully completed PowerPoint, a student version of the PowerPoint with sections to complete independently and some exam style questions.
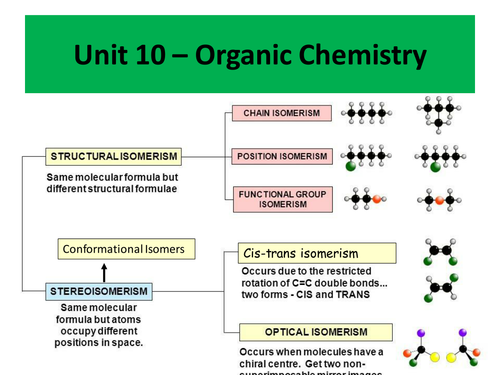
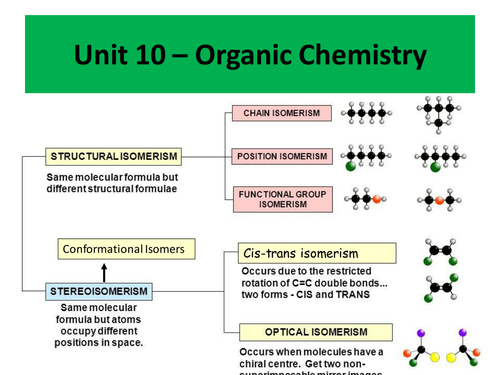
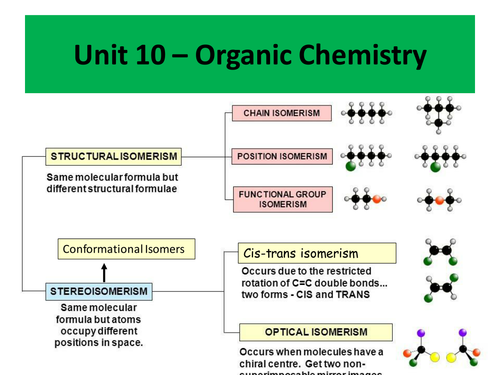
Topics covered include:
- Cis-trans isomerism
- Conformational isomerism
- Optical isomerism
- Optical Isomers and Plane-polarised light
- Racemic mixtures
- Diastereoisomers
Get this resource as part of a bundle and save up to 44%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
KS5 Acids and Bases, Redox and Organic Chemistry Schemes of Work
These 16 PowerPoints were planned as part of the IB scheme of work on Acids and Bases, Redox Chemistry and Organic Chemistry, and cover the necessary content for both the Standard and Higher Level topics. They would also be suitable for other post-16 courses. Included are fully completed PowerPoints, student versions of the PowerPoints with sections to complete independently and some exam style questions. Topics included are: \- What are acids and bases? \- Bronsted Lowry acids and bases (and conjugate acids and bases) \- Amphiprotic and amphoteric substances \- Lewis acids and bases \- Reactions of acids with metals, metal oxides, metal hydroxides, metal carbonates and metal hydrogencarbonates, bases and alkalis \- Making salts \- What is pH and how to calculate the pH of both acids and bases \- Using the dissociation constant of water to calculate pH \- Acid deposition - how it occurs and how it can be treated \- Calculations involving Ka, pKa, Kb, pKb, pH and pOH \- Using the relationships Kw = Ka x Kb and pKa + pKb = pKw \- Titration curves for titrations involving any combination of strong and weak acids and bases \- Indicators - how to select a suitable indicator for a titration \- How to calculate the pH of salt solutions \- Buffers - what are they, how are they made and how do they work (including calculations) Reduction and Oxidation Oxidation states and how to determine them Naming compounds using oxidation states Oxidising and reducing agents Half equations in molten substances Half equations in acidic solutions The activity series Redox titrations Winkler method to determine biochemical oxygen demand Voltaic Cells Electrolytic Cells Cell potentials The standard hydrogen electrode Ecell and spontaneity Working out cell potentials Polarity and direction of electron flow The electrochemical series Electrolysis of aqueous solutions The effect of the nature of electrodes on the products Electroplating Electrolysis of water Quantitative electrolysis \- Different kinds of formula e.g. molecular, empirical \- Alkanes \- Alkenes \- Compounds involving a benzene ring \- Homologous Series \- IUPAC nomenclature \- Naming halogenoalkanes \- Naming alcohols, ethers, aldehydes, ketones and carboxylic acids \- Esters \- Primary, secondary and tertiary alcohols, halogenoalkanes and amines \- Structural Isomerism \- Functional Group Isomerism \- Benzene and Aromatic Compounds \- Combustion of alkanes \- Reaction of alkanes with halogens \- Reactions of alkenes \- Addition polymerisation \- Oxidation of alcohols \- Nucleophilic Substitution mechanisms of primary, tertiary and secondary halogenoalkanes \- Factors affecting the rate of nucleophilic substitution \- Electrophilic Addition mechanisms \- Markovnikov´s Rule \- Electrophilic subtitution mechanisms \- Reduction Reactions \- Reaction pathways and synthetic routes \- Cis-trans isomerism \- Conformational isomerism \- Optical isomerism \- Optical Isomers and Plane-polarised light \- Racemic mixtures \- Diastereoisomers
Organic Chemistry
These five PowerPoints were planned as part of the IB scheme of work on Organic Chemistry, and cover the necessary content for both the Standard and Higher Level topics. It would also be suitable for other post-16 courses. Included are fully completed PowerPoints, student versions of the PowerPoints with sections to complete independently and some exam style questions. Topics included are: Organic Chemistry - Fundamentals and Functional GroupsEdit this resource In Chemistry by caverre 0 ratings These two PowerPoints were planned as part of the IB scheme of work on Organic Chemistry, and covers the necessary content for the Standard Level topics. It would also be suitable for other post-16 courses. Included are fully completed PowerPoints, student versions of the PowerPoints with sections to complete independently and some exam style questions. Topics covered: \- Different kinds of formula e.g. molecular, empirical \- Alkanes \- Alkenes \- Compounds involving a benzene ring \- Homologous Series \- IUPAC nomenclature \- Naming halogenoalkanes \- Naming alcohols, ethers, aldehydes, ketones and carboxylic acids \- Esters \- Primary, secondary and tertiary alcohols, halogenoalkanes and amines \- Structural Isomerism \- Functional Group Isomerism \- Benzene and Aromatic Compounds \- Combustion of alkanes \- Reaction of alkanes with halogens \- Reactions of alkenes \- Addition polymerisation \- Oxidation of alcohols \- Nucleophilic Substitution mechanisms of primary, tertiary and secondary halogenoalkanes \- Factors affecting the rate of nucleophilic substitution \- Electrophilic Addition mechanisms \- Markovnikov´s Rule \- Electrophilic subtitution mechanisms \- Reduction Reactions \- Reaction pathways and synthetic routes \- Cis-trans isomerism \- Conformational isomerism \- Optical isomerism \- Optical Isomers and Plane-polarised light \- Racemic mixtures \- Diastereoisomers
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.