

This fully resourced lesson teaches students what the difference between weak, strong, concentrated and dilute acids are. Students will also learn the link between H+ concentration and pH and how to calculate changes to pH using concentration and vice versa.
This lesson is scaffolded, designed for a mixed ability classroom and contains scaffolded worked examples with full answers to all activities.
This lesson is suitable for students studying AQA Trilogy Combined Science (higher and foundation) and AQA Triple chemistry (higher and foundation)
Lesson Objectives
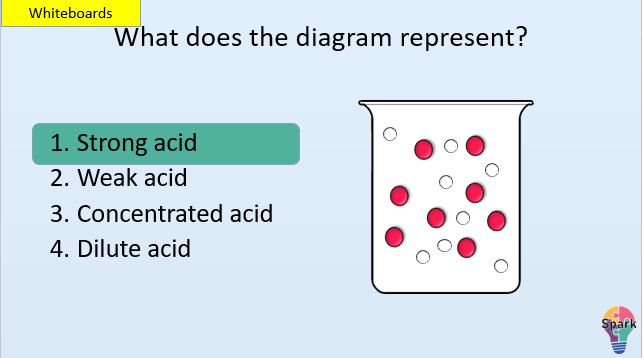
- State what a weak and strong acid are in terms of ionisation
- State what a concentrated acid and dilute acid are in terms of ions
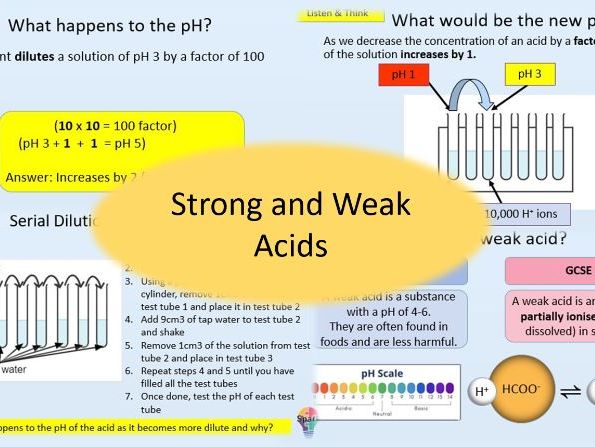
- Describe what happens to the pH of a solution as the concentration of H+ ions increases or decreases
- Calculate the new pH of a solution using dilution factors and vice versa
Lesson features
- AfL mini-whiteboard activities
- Scaffolded worked examples increasing in difficulty for students
- Student mini-practical (investigating pH with the serial dilution of a strong acid)
- Full answers integrated in slides
- Practice worksheet to summarise key learning, as well as past AQA Exam question (2024-2018) practice
Lesson Resource Contains
- Lesson Powerpoint (pptx.)
- Student worksheet (PDF)
- Student worksheet answers (PDF)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.