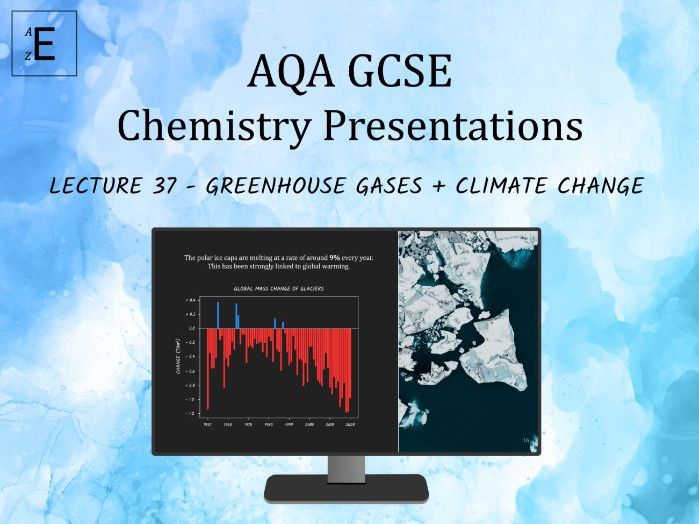
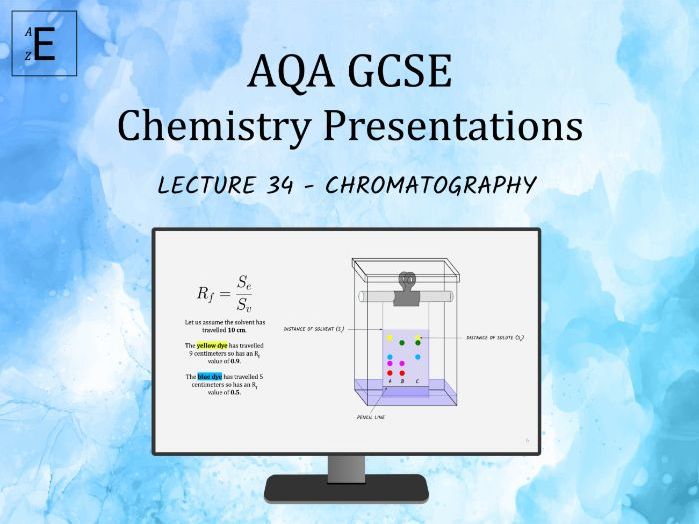
Empirical Tuition
We understand the importance of education. We offer tailored private tuition designed to maximise success at GCSE, A Level and beyond. Our tutors are experienced professionals, teachers and top-tier graduates with extensive knowledge in their subjects. On Tes we offer high quality board-specific resources. Please check back regularly as we are continually updating our stock.